Different Brain Regions are Infected with Fungi in Alzheimer's Disease
- PMID: 26468932
- PMCID: PMC4606562
- DOI: 10.1038/srep15015
Different Brain Regions are Infected with Fungi in Alzheimer's Disease
Abstract
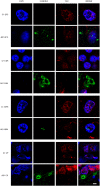
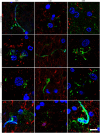
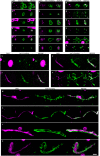
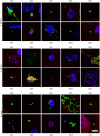
The possibility that Alzheimer's disease (AD) has a microbial aetiology has been proposed by several researchers. Here, we provide evidence that tissue from the central nervous system (CNS) of AD patients contain fungal cells and hyphae. Fungal material can be detected both intra- and extracellularly using specific antibodies against several fungi. Different brain regions including external frontal cortex, cerebellar hemisphere, entorhinal cortex/hippocampus and choroid plexus contain fungal material, which is absent in brain tissue from control individuals. Analysis of brain sections from ten additional AD patients reveals that all are infected with fungi. Fungal infection is also observed in blood vessels, which may explain the vascular pathology frequently detected in AD patients. Sequencing of fungal DNA extracted from frozen CNS samples identifies several fungal species. Collectively, our findings provide compelling evidence for the existence of fungal infection in the CNS from AD patients, but not in control individuals.
Figures
Similar articles
-
Direct visualization of fungal infection in brains from patients with Alzheimer's disease.J Alzheimers Dis. 2015;43(2):613-24. doi: 10.3233/JAD-141386. J Alzheimers Dis. 2015. PMID: 25125470
-
Identification of Fungal Species in Brain Tissue from Alzheimer's Disease by Next-Generation Sequencing.J Alzheimers Dis. 2017;58(1):55-67. doi: 10.3233/JAD-170058. J Alzheimers Dis. 2017. PMID: 28387676
-
Fungal infection in patients with Alzheimer's disease.J Alzheimers Dis. 2014;41(1):301-11. doi: 10.3233/JAD-132681. J Alzheimers Dis. 2014. PMID: 24614898
-
Role of microglia in fungal infections of the central nervous system.Virulence. 2017 Aug 18;8(6):705-718. doi: 10.1080/21505594.2016.1261789. Epub 2016 Nov 18. Virulence. 2017. PMID: 27858519 Free PMC article. Review.
-
[Significance of tau in the development of Alzheimer's disease].Brain Nerve. 2010 Jul;62(7):701-8. Brain Nerve. 2010. PMID: 20675874 Review. Japanese.
Cited by
-
Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease.Sci Transl Med. 2016 May 25;8(340):340ra72. doi: 10.1126/scitranslmed.aaf1059. Sci Transl Med. 2016. PMID: 27225182 Free PMC article.
-
Microbial involvement in Alzheimer disease development and progression.Mol Neurodegener. 2020 Jul 24;15(1):42. doi: 10.1186/s13024-020-00378-4. Mol Neurodegener. 2020. PMID: 32709243 Free PMC article. Review.
-
Morphology of blood microbiota in healthy individuals assessed by light and electron microscopy.Front Cell Infect Microbiol. 2023 Jan 18;12:1091341. doi: 10.3389/fcimb.2022.1091341. eCollection 2022. Front Cell Infect Microbiol. 2023. PMID: 36741978 Free PMC article.
-
When the infectious environment meets the AD brain.Mol Neurodegener. 2022 Aug 19;17(1):53. doi: 10.1186/s13024-022-00559-3. Mol Neurodegener. 2022. PMID: 35986296 Free PMC article. Review.
-
The Physiological Roles of Amyloid-β Peptide Hint at New Ways to Treat Alzheimer's Disease.Front Aging Neurosci. 2018 Apr 25;10:118. doi: 10.3389/fnagi.2018.00118. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29922148 Free PMC article. Review.
References
-
- Mackenzie I. R., Neumann M., Cairns N. J., Munoz D. G. & Isaacs A. M. Novel types of frontotemporal lobar degeneration: beyond tau and TDP-43. J Mol Neurosci 45, 402–408 (2011). - PubMed
-
- Roussel B. D. et al. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 12, 105–118 (2013). - PubMed
-
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4, 49–60 (2003). - PubMed
-
- Krstic D. & Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol 9, 25–34 (2013). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

