The Frank-Starling mechanism involves deceleration of cross-bridge kinetics and is preserved in failing human right ventricular myocardium
- PMID: 26453335
- PMCID: PMC4698421
- DOI: 10.1152/ajpheart.00685.2015
The Frank-Starling mechanism involves deceleration of cross-bridge kinetics and is preserved in failing human right ventricular myocardium
Abstract
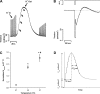
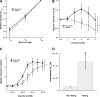
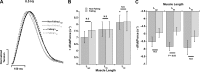
Cross-bridge cycling rate is an important determinant of cardiac output, and its alteration can potentially contribute to reduced output in heart failure patients. Additionally, animal studies suggest that this rate can be regulated by muscle length. The purpose of this study was to investigate cross-bridge cycling rate and its regulation by muscle length under near-physiological conditions in intact right ventricular muscles of nonfailing and failing human hearts. We acquired freshly explanted nonfailing (n = 9) and failing (n = 10) human hearts. All experiments were performed on intact right ventricular cardiac trabeculae (n = 40) at physiological temperature and near the normal heart rate range. The failing myocardium showed the typical heart failure phenotype: a negative force-frequency relationship and β-adrenergic desensitization (P < 0.05), indicating the expected pathological myocardium in the right ventricles. We found that there exists a length-dependent regulation of cross-bridge cycling kinetics in human myocardium. Decreasing muscle length accelerated the rate of cross-bridge reattachment (ktr) in both nonfailing and failing myocardium (P < 0.05) equally; there were no major differences between nonfailing and failing myocardium at each respective length (P > 0.05), indicating that this regulatory mechanism is preserved in heart failure. Length-dependent assessment of twitch kinetics mirrored these findings; normalized dF/dt slowed down with increasing length of the muscle and was virtually identical in diseased tissue. This study shows for the first time that muscle length regulates cross-bridge kinetics in human myocardium under near-physiological conditions and that those kinetics are preserved in the right ventricular tissues of heart failure patients.
Keywords: cross-bridge cycling kinetics; heart failure; muscle length; relaxation; trabeculae.
Copyright © 2015 the American Physiological Society.
Figures
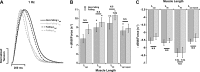
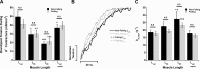
Similar articles
-
Impact of heart rate on cross-bridge cycling kinetics in failing and nonfailing human myocardium.Am J Physiol Heart Circ Physiol. 2019 Sep 1;317(3):H640-H647. doi: 10.1152/ajpheart.00163.2019. Epub 2019 Jul 26. Am J Physiol Heart Circ Physiol. 2019. PMID: 31347914 Free PMC article.
-
Alterations of cross-bridge kinetics in human atrial and ventricular myocardium.Cardiovasc Res. 1998 Dec;40(3):580-90. doi: 10.1016/s0008-6363(98)00164-3. Cardiovasc Res. 1998. PMID: 10070500
-
Insights into length-dependent regulation of cardiac cross-bridge cycling kinetics in human myocardium.Arch Biochem Biophys. 2016 Jul 1;601:48-55. doi: 10.1016/j.abb.2016.02.005. Epub 2016 Feb 18. Arch Biochem Biophys. 2016. PMID: 26854725 Free PMC article.
-
Calcium cycling proteins and force-frequency relationship in heart failure.Basic Res Cardiol. 1996;91 Suppl 2:17-22. doi: 10.1007/BF00795357. Basic Res Cardiol. 1996. PMID: 8957539 Review.
-
Is contractility depressed in the failing human heart?Cardiovasc Drugs Ther. 1995 Aug;9(4):581-7. doi: 10.1007/BF00878090. Cardiovasc Drugs Ther. 1995. PMID: 8547208 Review.
Cited by
-
Myocardial relaxation in human heart failure: Why sarcomere kinetics should be center-stage.Arch Biochem Biophys. 2019 Jan;661:145-148. doi: 10.1016/j.abb.2018.11.011. Epub 2018 Nov 14. Arch Biochem Biophys. 2019. PMID: 30447209 Free PMC article. Review.
-
Highly variable contractile performance correlates with myocyte content in trabeculae from failing human hearts.Sci Rep. 2018 Feb 13;8(1):2957. doi: 10.1038/s41598-018-21199-y. Sci Rep. 2018. PMID: 29440728 Free PMC article.
-
Increased cross-bridge recruitment contributes to transient increase in force generation beyond maximal capacity in human myocardium.J Mol Cell Cardiol. 2018 Jan;114:116-123. doi: 10.1016/j.yjmcc.2017.11.007. Epub 2017 Nov 12. J Mol Cell Cardiol. 2018. PMID: 29141185 Free PMC article.
-
Length-Dependent Prolongation of Force Relaxation Is Unaltered by Delay of Intracellular Calcium Decline in Early-Stage Rabbit Right Ventricular Hypertrophy.Front Physiol. 2017 Dec 4;8:945. doi: 10.3389/fphys.2017.00945. eCollection 2017. Front Physiol. 2017. PMID: 29255420 Free PMC article.
-
The Need for Speed: Mice, Men, and Myocardial Kinetic Reserve.Circ Res. 2016 Jul 22;119(3):418-21. doi: 10.1161/CIRCRESAHA.116.309126. Circ Res. 2016. PMID: 27458197 Free PMC article. Review.
References
-
- Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. - PubMed
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic, 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

