Metabolic reprogramming: the emerging concept and associated therapeutic strategies
- PMID: 26445347
- PMCID: PMC4595070
- DOI: 10.1186/s13046-015-0221-y
Metabolic reprogramming: the emerging concept and associated therapeutic strategies
Abstract
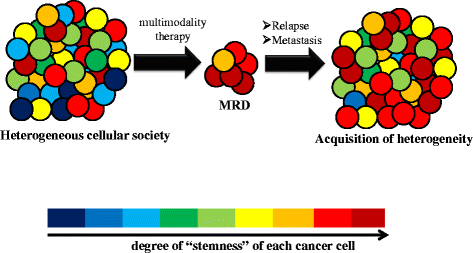
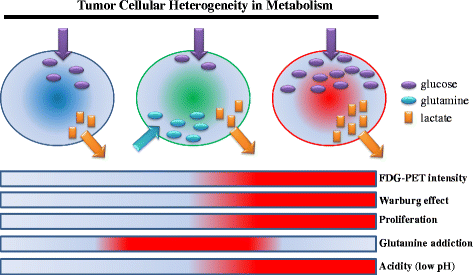
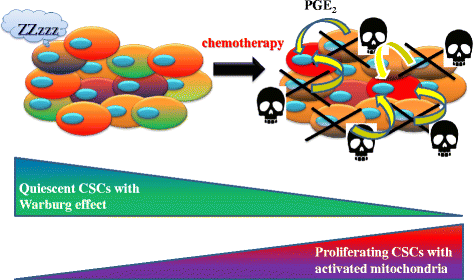
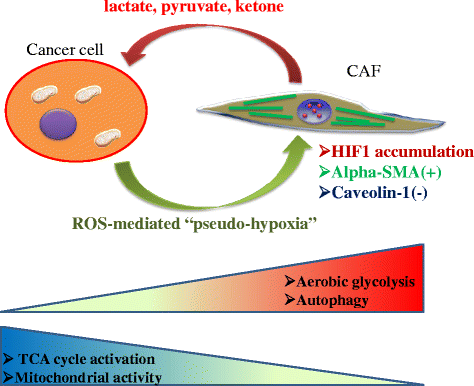
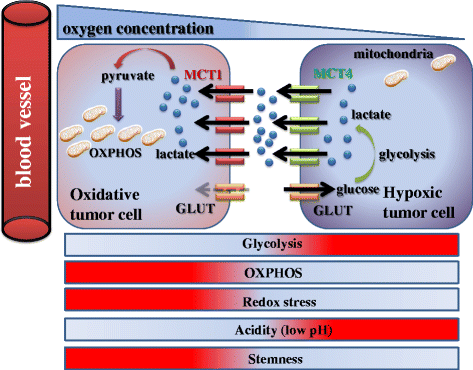
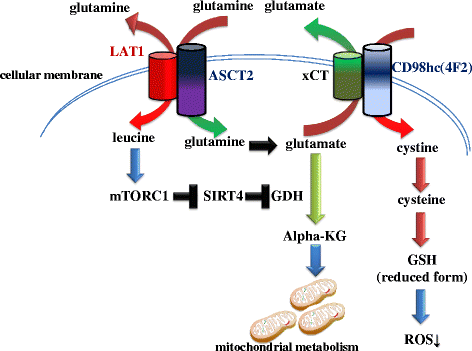
Tumor tissue is composed of cancer cells and surrounding stromal cells with diverse genetic/epigenetic backgrounds, a situation known as intra-tumoral heterogeneity. Cancer cells are surrounded by a totally different microenvironment than that of normal cells; consequently, tumor cells must exhibit rapidly adaptive responses to hypoxia and hypo-nutrient conditions. This phenomenon of changes of tumor cellular bioenergetics, called "metabolic reprogramming", has been recognized as one of 10 hallmarks of cancer. Metabolic reprogramming is required for both malignant transformation and tumor development, including invasion and metastasis. Although the Warburg effect has been widely accepted as a common feature of metabolic reprogramming, accumulating evidence has revealed that tumor cells depend on mitochondrial metabolism as well as aerobic glycolysis. Remarkably, cancer-associated fibroblasts in tumor stroma tend to activate both glycolysis and autophagy in contrast to neighboring cancer cells, which leads to a reverse Warburg effect. Heterogeneity of monocarboxylate transporter expression reflects cellular metabolic heterogeneity with respect to the production and uptake of lactate. In tumor tissue, metabolic heterogeneity induces metabolic symbiosis, which is responsible for adaptation to drastic changes in the nutrient microenvironment resulting from chemotherapy. In addition, metabolic heterogeneity is responsible for the failure to induce the same therapeutic effect against cancer cells as a whole. In particular, cancer stem cells exhibit several biological features responsible for resistance to conventional anti-tumor therapies. Consequently, cancer stem cells tend to form minimal residual disease after chemotherapy and exhibit metastatic potential with additional metabolic reprogramming. This type of altered metabolic reprogramming leads to adaptive/acquired resistance to anti-tumor therapy. Collectively, complex and dynamic metabolic reprogramming should be regarded as a reflection of the "robustness" of tumor cells against unfavorable conditions. This review focuses on the concept of metabolic reprogramming in heterogeneous tumor tissue, and further emphasizes the importance of developing novel therapeutic strategies based on drug repositioning.
Figures
Similar articles
-
Mitochondria and cancer chemoresistance.Biochim Biophys Acta Bioenerg. 2017 Aug;1858(8):686-699. doi: 10.1016/j.bbabio.2017.01.012. Epub 2017 Feb 1. Biochim Biophys Acta Bioenerg. 2017. PMID: 28161329 Review.
-
Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function.Semin Oncol. 2014 Apr;41(2):195-216. doi: 10.1053/j.seminoncol.2014.03.002. Epub 2014 Mar 5. Semin Oncol. 2014. PMID: 24787293 Review.
-
[Even the Warburg effect can be oxidized: metabolic cooperation and tumor development].Med Sci (Paris). 2018 Aug-Sep;34(8-9):701-708. doi: 10.1051/medsci/20183408017. Epub 2018 Sep 19. Med Sci (Paris). 2018. PMID: 30230466 Review. French.
-
The dichotomous role of the glycolytic metabolism pathway in cancer metastasis: Interplay with the complex tumor microenvironment and novel therapeutic strategies.Semin Cancer Biol. 2020 Feb;60:238-248. doi: 10.1016/j.semcancer.2019.08.025. Epub 2019 Aug 21. Semin Cancer Biol. 2020. PMID: 31445217 Review.
-
Linking metabolic reprogramming to therapy resistance in cancer.Biochim Biophys Acta Rev Cancer. 2017 Aug;1868(1):1-6. doi: 10.1016/j.bbcan.2016.12.004. Epub 2017 Jan 5. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28065746 Review.
Cited by
-
Metabolic reprogramming of the tumor immune microenvironment in ovarian cancer: A novel orientation for immunotherapy.Front Immunol. 2022 Oct 14;13:1030831. doi: 10.3389/fimmu.2022.1030831. eCollection 2022. Front Immunol. 2022. PMID: 36311734 Free PMC article. Review.
-
Targeting cancer-cell mitochondria and metabolism to improve radiotherapy response.Transl Oncol. 2021 Jan;14(1):100905. doi: 10.1016/j.tranon.2020.100905. Epub 2020 Oct 14. Transl Oncol. 2021. PMID: 33069104 Free PMC article. Review.
-
T-Cell Metabolic Reprogramming in Atherosclerosis.Biomedicines. 2024 Aug 14;12(8):1844. doi: 10.3390/biomedicines12081844. Biomedicines. 2024. PMID: 39200308 Free PMC article. Review.
-
Metronomic Chemotherapy Modulates Clonal Interactions to Prevent Drug Resistance in Non-Small Cell Lung Cancer.Cancers (Basel). 2021 May 7;13(9):2239. doi: 10.3390/cancers13092239. Cancers (Basel). 2021. PMID: 34066944 Free PMC article.
-
Dataset for analysis of metabolic pathways and their reversibility associated with anti-proliferative effect of metformin in liver cancer cells.Data Brief. 2024 May 28;55:110562. doi: 10.1016/j.dib.2024.110562. eCollection 2024 Aug. Data Brief. 2024. PMID: 38952952 Free PMC article.
References
-
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

