Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells
- PMID: 26431332
- PMCID: PMC4592227
- DOI: 10.1371/journal.ppat.1005195
Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells
Abstract
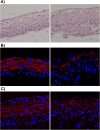
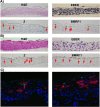
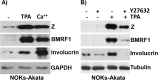
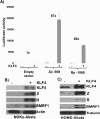
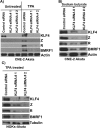
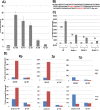
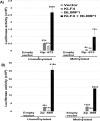
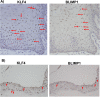
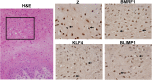
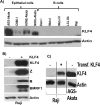
Epstein-Barr virus (EBV) is a human herpesvirus associated with B-cell and epithelial cell malignancies. EBV lytically infects normal differentiated oral epithelial cells, where it causes a tongue lesion known as oral hairy leukoplakia (OHL) in immunosuppressed patients. However, the cellular mechanism(s) that enable EBV to establish exclusively lytic infection in normal differentiated oral epithelial cells are not currently understood. Here we show that a cellular transcription factor known to promote epithelial cell differentiation, KLF4, induces differentiation-dependent lytic EBV infection by binding to and activating the two EBV immediate-early gene (BZLF1 and BRLF1) promoters. We demonstrate that latently EBV-infected, telomerase-immortalized normal oral keratinocyte (NOKs) cells undergo lytic viral reactivation confined to the more differentiated cell layers in organotypic raft culture. Furthermore, we show that endogenous KLF4 expression is required for efficient lytic viral reactivation in response to phorbol ester and sodium butyrate treatment in several different EBV-infected epithelial cell lines, and that the combination of KLF4 and another differentiation-dependent cellular transcription factor, BLIMP1, is highly synergistic for inducing lytic EBV infection. We confirm that both KLF4 and BLIMP1 are expressed in differentiated, but not undifferentiated, epithelial cells in normal tongue tissue, and show that KLF4 and BLIMP1 are both expressed in a patient-derived OHL lesion. In contrast, KLF4 protein is not detectably expressed in B cells, where EBV normally enters latent infection, although KLF4 over-expression is sufficient to induce lytic EBV reactivation in Burkitt lymphoma cells. Thus, KLF4, together with BLIMP1, plays a critical role in mediating lytic EBV reactivation in epithelial cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Differentiation-Dependent LMP1 Expression Is Required for Efficient Lytic Epstein-Barr Virus Reactivation in Epithelial Cells.J Virol. 2017 Mar 29;91(8):e02438-16. doi: 10.1128/JVI.02438-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28179525 Free PMC article.
-
Cellular differentiation regulator BLIMP1 induces Epstein-Barr virus lytic reactivation in epithelial and B cells by activating transcription from both the R and Z promoters.J Virol. 2015 Feb;89(3):1731-43. doi: 10.1128/JVI.02781-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410866 Free PMC article.
-
ΔNp63α promotes Epstein-Barr virus latency in undifferentiated epithelial cells.PLoS Pathog. 2021 Nov 8;17(11):e1010045. doi: 10.1371/journal.ppat.1010045. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34748616 Free PMC article.
-
EBV Infection and Glucose Metabolism in Nasopharyngeal Carcinoma.Adv Exp Med Biol. 2017;1018:75-90. doi: 10.1007/978-981-10-5765-6_6. Adv Exp Med Biol. 2017. PMID: 29052133 Review.
-
Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence?Adv Cancer Res. 2000;79:175-200. doi: 10.1016/s0065-230x(00)79006-3. Adv Cancer Res. 2000. PMID: 10818681 Review.
Cited by
-
Long non-coding RNAs in Epstein-Barr virus-related cancer.Cancer Cell Int. 2021 May 25;21(1):278. doi: 10.1186/s12935-021-01986-w. Cancer Cell Int. 2021. PMID: 34034760 Free PMC article. Review.
-
Identification of an N6-methyladenosine-mediated positive feedback loop that promotes Epstein-Barr virus infection.J Biol Chem. 2021 Jan-Jun;296:100547. doi: 10.1016/j.jbc.2021.100547. Epub 2021 Mar 16. J Biol Chem. 2021. PMID: 33741341 Free PMC article.
-
Post-Transcriptional Regulation of KLF4 by High-Risk Human Papillomaviruses Is Necessary for the Differentiation-Dependent Viral Life Cycle.PLoS Pathog. 2016 Jul 7;12(7):e1005747. doi: 10.1371/journal.ppat.1005747. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27386862 Free PMC article.
-
A distinct isoform of lymphoid enhancer binding factor 1 (LEF1) epigenetically restricts EBV reactivation to maintain viral latency.PLoS Pathog. 2023 Dec 19;19(12):e1011873. doi: 10.1371/journal.ppat.1011873. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38113273 Free PMC article.
-
New insight into Epstein-Barr virus infection using models of stratified epithelium.PLoS Pathog. 2023 Jan 11;19(1):e1011040. doi: 10.1371/journal.ppat.1011040. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36630458 Free PMC article.
References
-
- Rickinson A. B., Kieff Elliot. Epstein-Barr Virus Fields Virology. 5th ed. Philadelphia: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007. pp. 2655–2700.
-
- Zur Hausen H, Schulte-Holthausen H, Klein G, Henle W, Henle G, Clifford P, et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228: 1056–1058. - PubMed
-
- Kieff Elliot D, Rickinson A. B. Epstein-Barr Virus and Its Replication Fields Virology. 5th ed. Philadelphia: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007. pp. 2603–2654.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

