Molecular switches under TGFβ signalling during progression from cardiac hypertrophy to heart failure
- PMID: 26431212
- PMCID: PMC4813390
- DOI: 10.1111/bph.13344
Molecular switches under TGFβ signalling during progression from cardiac hypertrophy to heart failure
Abstract
Cardiac hypertrophy is a mechanism to compensate for increased cardiac work load, that is, after myocardial infarction or upon pressure overload. However, in the long run cardiac hypertrophy is a prevailing risk factor for the development of heart failure. During pathological remodelling processes leading to heart failure, decompensated hypertrophy, death of cardiomyocytes by apoptosis or necroptosis and fibrosis as well as a progressive dysfunction of cardiomyocytes are apparent. Interestingly, the induction of hypertrophy, cell death or fibrosis is mediated by similar signalling pathways. Therefore, tiny changes in the signalling cascade are able to switch physiological cardiac remodelling to the development of heart failure. In the present review, we will describe examples of these molecular switches that change compensated hypertrophy to the development of heart failure and will focus on the importance of the signalling cascades of the TGFβ superfamily in this process. In this context, potential therapeutic targets for pharmacological interventions that could attenuate the progression of heart failure will be discussed.
© 2015 The British Pharmacological Society.
Figures
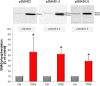
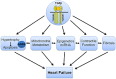
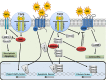
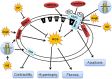
Similar articles
-
Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart.Cardiovasc Res. 2011 Feb 1;89(2):265-72. doi: 10.1093/cvr/cvq308. Epub 2010 Sep 28. Cardiovasc Res. 2011. PMID: 20880837 Review.
-
The microRNA-15 family inhibits the TGFβ-pathway in the heart.Cardiovasc Res. 2014 Oct 1;104(1):61-71. doi: 10.1093/cvr/cvu184. Epub 2014 Aug 7. Cardiovasc Res. 2014. PMID: 25103110
-
NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure.Cardiovasc Res. 2006 Jul 15;71(2):208-15. doi: 10.1016/j.cardiores.2006.03.016. Epub 2006 Mar 27. Cardiovasc Res. 2006. PMID: 16631149 Review.
-
A direct interaction between TGFbeta activated kinase 1 and the TGFbeta type II receptor: implications for TGFbeta signalling and cardiac hypertrophy.Cardiovasc Res. 2006 Feb 1;69(2):432-9. doi: 10.1016/j.cardiores.2005.11.007. Epub 2005 Dec 19. Cardiovasc Res. 2006. PMID: 16360132
-
Loss of AKAP150 promotes pathological remodelling and heart failure propensity by disrupting calcium cycling and contractile reserve.Cardiovasc Res. 2017 Feb;113(2):147-159. doi: 10.1093/cvr/cvw221. Epub 2016 Nov 17. Cardiovasc Res. 2017. PMID: 27856611 Free PMC article.
Cited by
-
LOXL2 silencing suppresses angiotensin II-induced cardiac hypertrophy through the EMT process and TGF-β1/Smad3/NF-κB pathway.Iran J Basic Med Sci. 2022 Aug;25(8):964-969. doi: 10.22038/IJBMS.2022.63338.13981. Iran J Basic Med Sci. 2022. PMID: 36159334 Free PMC article.
-
ZYZ-168 alleviates cardiac fibrosis after myocardial infarction through inhibition of ERK1/2-dependent ROCK1 activation.Sci Rep. 2017 Mar 7;7:43242. doi: 10.1038/srep43242. Sci Rep. 2017. PMID: 28266583 Free PMC article.
-
The role of pyroptosis in inflammatory diseases.Front Cell Dev Biol. 2023 May 12;11:1173235. doi: 10.3389/fcell.2023.1173235. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37250902 Free PMC article. Review.
-
Status of β1-Adrenoceptor Signal Transduction System in Cardiac Hypertrophy and Heart Failure.Rev Cardiovasc Med. 2023 Sep 21;24(9):264. doi: 10.31083/j.rcm2409264. eCollection 2023 Sep. Rev Cardiovasc Med. 2023. PMID: 39076390 Free PMC article. Review.
-
Gene module regulation in dilated cardiomyopathy and the role of Na/K-ATPase.PLoS One. 2022 Jul 28;17(7):e0272117. doi: 10.1371/journal.pone.0272117. eCollection 2022. PLoS One. 2022. PMID: 35901050 Free PMC article.
References
-
- Abbate A, Arena R, Abouzaki N, Van Tassell BW, Canada J, Shah K et al. (2015). Heart failure with preserved ejection fraction: refocusing on diastole. Int J Cardiol 179: 430–440. doi:10.1016/j.ijcard.2014.11.106. - DOI - PubMed
-
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013a). The Concise Guide to PHARMACOLOGY 2013/14: G protein‐coupled receptors. Br J Pharmacol 170: 1459–1581. doi:10.1111/bph.12445. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

