In vitro and in vivo correlates of physiological and neoplastic human Fallopian tube stem cells
- PMID: 26415052
- PMCID: PMC4895925
- DOI: 10.1002/path.4649
In vitro and in vivo correlates of physiological and neoplastic human Fallopian tube stem cells
Abstract
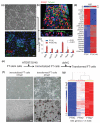
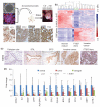
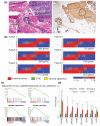
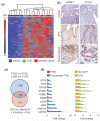
High-grade serous cancer (HGSC) progresses to advanced stages without symptoms and the 5-year survival rate is a dismal 30%. Recent studies of ovaries and Fallopian tubes in patients with BRCA1 or BRCA2 mutations have documented a pre-metastatic intramucosal neoplasm that is found almost exclusively in the Fallopian tube, termed 'serous tubal intraepithelial carcinoma' or STIC. Moreover, other proliferations, termed p53 signatures, secretory cell outgrowths (SCOUTs), and lower-grade serous tubal intraepithelial neoplasms (STINs) fall short of STIC but share similar alterations in expression, in keeping with an underpinning of genomic disturbances involved in, or occurring in parallel with, serous carcinogenesis. To gain insight into the cellular origins of this unique tubal pathway to high-grade serous cancer, we cloned and both immortalized and transformed Fallopian tube stem cells (FTSCs). We demonstrated that pedigrees of FTSCs were capable of multipotent differentiation and that the tumours derived from transformed FTSCs shared the histological and molecular features of HGSC. We also demonstrated that altered expression of some biomarkers seen in transformed FTSCs and HGSCs (stathmin, EZH2, CXCR4, CXCL12, and FOXM1) could be seen as well in immortalized cells and their in vivo counterparts SCOUTs and STINs. Thus, a whole-genome transcriptome analysis comparing FTSCs, immortalized FTSCs, and transformed FTSCs showed a clear molecular progression sequence that is recapitulated by the spectrum of accumulated perturbations characterizing the range of proliferations seen in vivo. Biomarkers unique to STIC relative to normal tubal epithelium provide a basis for novel detection approaches to early HGSC, but must be viewed critically given their potential expression in lesser proliferations. Perturbations shared by both immortalized and transformed FTSCs may provide unique early targets for prevention strategies. Central to these efforts has been the ability to clone and perpetuate multipotent FTSCs.
Keywords: Fallopian tubes; cell culture; neoplasia; ovary.
Copyright © 2015 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Figures
Similar articles
-
Evidence for lineage continuity between early serous proliferations (ESPs) in the Fallopian tube and disseminated high-grade serous carcinomas.J Pathol. 2018 Nov;246(3):344-351. doi: 10.1002/path.5145. Epub 2018 Sep 27. J Pathol. 2018. PMID: 30043522
-
[Morphologic features of fallopian tubal epithelium in pelvic high-grade serous carcinoma].Zhonghua Bing Li Xue Za Zhi. 2017 Aug 8;46(8):542-547. doi: 10.3760/cma.j.issn.0529-5807.2017.08.005. Zhonghua Bing Li Xue Za Zhi. 2017. PMID: 28810294 Chinese.
-
The fallopian tube, "precursor escape" and narrowing the knowledge gap to the origins of high-grade serous carcinoma.Gynecol Oncol. 2019 Feb;152(2):426-433. doi: 10.1016/j.ygyno.2018.11.033. Epub 2018 Nov 30. Gynecol Oncol. 2019. PMID: 30503267 Review.
-
Incidental serous tubal intraepithelial carcinoma and early invasive serous carcinoma in the nonprophylactic setting: analysis of a case series.Am J Surg Pathol. 2015 Apr;39(4):442-53. doi: 10.1097/PAS.0000000000000352. Am J Surg Pathol. 2015. PMID: 25517955
-
Advances in serous tubal intraepithelial carcinoma: correlation with high grade serous carcinoma and ovarian carcinogenesis.Int J Clin Exp Pathol. 2014 Feb 15;7(3):848-57. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24696706 Free PMC article. Review.
Cited by
-
RAD21 amplification epigenetically suppresses interferon signaling to promote immune evasion in ovarian cancer.J Clin Invest. 2022 Nov 15;132(22):e159628. doi: 10.1172/JCI159628. J Clin Invest. 2022. PMID: 36201246 Free PMC article.
-
Fallopian tube lesions as potential precursors of early ovarian cancer: a comprehensive proteomic analysis.Cell Death Dis. 2023 Sep 30;14(9):644. doi: 10.1038/s41419-023-06165-5. Cell Death Dis. 2023. PMID: 37775701 Free PMC article.
-
Ovarian Cancer, Cancer Stem Cells and Current Treatment Strategies: A Potential Role of Magmas in the Current Treatment Methods.Cells. 2020 Mar 14;9(3):719. doi: 10.3390/cells9030719. Cells. 2020. PMID: 32183385 Free PMC article. Review.
-
PAX2 function, regulation and targeting in fallopian tube-derived high-grade serous ovarian cancer.Oncogene. 2017 May 25;36(21):3015-3024. doi: 10.1038/onc.2016.455. Epub 2016 Dec 19. Oncogene. 2017. PMID: 27991925 Free PMC article.
-
TET1 reprograms the epithelial ovarian cancer epigenome and reveals casein kinase 2α as a therapeutic target.J Pathol. 2019 Jul;248(3):363-376. doi: 10.1002/path.5266. Epub 2019 Apr 23. J Pathol. 2019. PMID: 30883733 Free PMC article.
References
-
- Auersperg N, Wong AS, Choi KC, et al. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–288. - PubMed
-
- Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. - PubMed
-
- Marquez RT, Baggerly KA, Patterson AP, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–6126. - PubMed
-
- Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

