Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells
- PMID: 26413868
- PMCID: PMC4639974
- DOI: 10.1172/JCI82314
Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells
Abstract
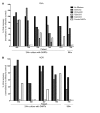
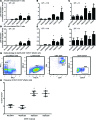
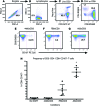
Enhancement of HIV-specific immunity is likely required to eliminate latent HIV infection. Here, we have developed an immunotherapeutic modality aimed to improve T cell-mediated clearance of HIV-1-infected cells. Specifically, we employed Dual-Affinity Re-Targeting (DART) proteins, which are bispecific, antibody-based molecules that can bind 2 distinct cell-surface molecules simultaneously. We designed DARTs with a monovalent HIV-1 envelope-binding (Env-binding) arm that was derived from broadly binding, antibody-dependent cellular cytotoxicity-mediating antibodies known to bind to HIV-infected target cells coupled to a monovalent CD3 binding arm designed to engage cytolytic effector T cells (referred to as HIVxCD3 DARTs). Thus, these DARTs redirected polyclonal T cells to specifically engage with and kill Env-expressing cells, including CD4+ T cells infected with different HIV-1 subtypes, thereby obviating the requirement for HIV-specific immunity. Using lymphocytes from patients on suppressive antiretroviral therapy (ART), we demonstrated that DARTs mediate CD8+ T cell clearance of CD4+ T cells that are superinfected with the HIV-1 strain JR-CSF or infected with autologous reservoir viruses isolated from HIV-infected-patient resting CD4+ T cells. Moreover, DARTs mediated CD8+ T cell clearance of HIV from resting CD4+ T cell cultures following induction of latent virus expression. Combined with HIV latency reversing agents, HIVxCD3 DARTs have the potential to be effective immunotherapeutic agents to clear latent HIV-1 reservoirs in HIV-infected individuals.
Figures
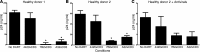
Similar articles
-
Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells.PLoS Pathog. 2015 Nov 5;11(11):e1005233. doi: 10.1371/journal.ppat.1005233. eCollection 2015. PLoS Pathog. 2015. PMID: 26539983 Free PMC article.
-
Chimeric Antigen Receptor T Cells Guided by the Single-Chain Fv of a Broadly Neutralizing Antibody Specifically and Effectively Eradicate Virus Reactivated from Latency in CD4+ T Lymphocytes Isolated from HIV-1-Infected Individuals Receiving Suppressive Combined Antiretroviral Therapy.J Virol. 2016 Oct 14;90(21):9712-9724. doi: 10.1128/JVI.00852-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535056 Free PMC article.
-
Elimination of SHIV Infected Cells by Combinations of Bispecific HIVxCD3 DART® Molecules.Front Immunol. 2021 Aug 13;12:710273. doi: 10.3389/fimmu.2021.710273. eCollection 2021. Front Immunol. 2021. PMID: 34484212 Free PMC article.
-
Therapeutic Targeting of HIV Reservoirs: How to Give T Cells a New Direction.Front Immunol. 2018 Dec 4;9:2861. doi: 10.3389/fimmu.2018.02861. eCollection 2018. Front Immunol. 2018. PMID: 30564246 Free PMC article. Review.
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
Cited by
-
Anti-viral efficacy of a next-generation CD4-binding site bNAb in SHIV-infected animals in the absence of anti-drug antibody responses.iScience. 2022 Sep 5;25(10):105067. doi: 10.1016/j.isci.2022.105067. eCollection 2022 Oct 21. iScience. 2022. PMID: 36157588 Free PMC article.
-
Curing HIV: Seeking to Target and Clear Persistent Infection.Cell. 2020 Apr 2;181(1):189-206. doi: 10.1016/j.cell.2020.03.005. Epub 2020 Mar 26. Cell. 2020. PMID: 32220311 Free PMC article. Review.
-
Proviral Latency, Persistent Human Immunodeficiency Virus Infection, and the Development of Latency Reversing Agents.J Infect Dis. 2017 Mar 15;215(suppl_3):S111-S118. doi: 10.1093/infdis/jiw618. J Infect Dis. 2017. PMID: 28520964 Free PMC article. Review.
-
The making of bispecific antibodies.MAbs. 2017 Feb/Mar;9(2):182-212. doi: 10.1080/19420862.2016.1268307. MAbs. 2017. PMID: 28071970 Free PMC article. Review.
-
Humanized Mouse Models for Human Immunodeficiency Virus Infection.Annu Rev Virol. 2017 Sep 29;4(1):393-412. doi: 10.1146/annurev-virology-101416-041703. Epub 2017 Jul 26. Annu Rev Virol. 2017. PMID: 28746819 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

