A Dietary Supplement Containing Cinnamon, Chromium and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-Diabetic Subjects: A Randomized, Placebo-Controlled Trial
- PMID: 26406981
- PMCID: PMC4583280
- DOI: 10.1371/journal.pone.0138646
A Dietary Supplement Containing Cinnamon, Chromium and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-Diabetic Subjects: A Randomized, Placebo-Controlled Trial
Erratum in
-
Correction: A Dietary Supplement Containing Cinnamon, Chromium and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-Diabetic Subjects: A Randomized, Placebo-Controlled Trial.PLoS One. 2015 Dec 14;10(12):e0145315. doi: 10.1371/journal.pone.0145315. eCollection 2015. PLoS One. 2015. PMID: 26661459 Free PMC article. No abstract available.
Abstract
Background: Preventing or slowing the progression of prediabetes to diabetes is a major therapeutic issue.
Objectives: Our aim was to evaluate the effects of 4-month treatment with a dietary supplement containing cinnamon, chromium and carnosine in moderately obese or overweight pre-diabetic subjects, the primary outcome being change in fasting plasma glucose (FPG) level. Other parameters of plasma glucose homeostasis, lipid profile, adiposity and inflammatory markers were also assessed.
Methods: In a randomized, double-blind, placebo-controlled study, 62 subjects with a FPG level ranging from 5.55 to 7 mmol/L and a body mass index ≥ 25 kg/m(2), unwilling to change their dietary and physical activity habits, were allocated to receive a 4-month treatment with either 1.2 g/day of the dietary supplement or placebo. Patients were followed up until 6 months post-randomization.
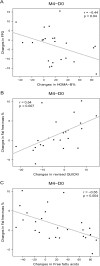
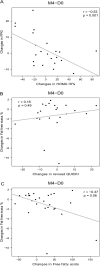
Results: Four-month treatment with the dietary supplement decreased FPG compared to placebo (-0.24 ± 0.50 vs +0.12 ± 0.59 mmol/L, respectively, p = 0.02), without detectable significant changes in HbA1c. Insulin sensitivity markers, plasma insulin, plasma lipids and inflammatory markers did not differ between the treatment groups. Although there were no significant differences in changes in body weight and energy or macronutrient intakes between the two groups, fat-free mass (%) increased with the dietary supplement compared to placebo (p = 0.02). Subjects with a higher FPG level and a milder inflammatory state at baseline benefited most from the dietary supplement.
Conclusions: Four-month treatment with a dietary supplement containing cinnamon, chromium and carnosine decreased FPG and increased fat-free mass in overweight or obese pre-diabetic subjects. These beneficial effects might open up new avenues in the prevention of diabetes.
Trial registration: ClinicalTrials.gov NCT01530685.
Conflict of interest statement
Figures
Similar articles
-
Cinnamomum zeylanicum (Ceylon cinnamon) as a potential pharmaceutical agent for type-2 diabetes mellitus: study protocol for a randomized controlled trial.Trials. 2017 Sep 29;18(1):446. doi: 10.1186/s13063-017-2192-0. Trials. 2017. PMID: 28962661 Free PMC article. Clinical Trial.
-
Does supplementation with carnosine improve cardiometabolic health and cognitive function in patients with pre-diabetes and type 2 diabetes? study protocol for a randomised, double-blind, placebo-controlled trial.BMJ Open. 2017 Sep 1;7(9):e017691. doi: 10.1136/bmjopen-2017-017691. BMJ Open. 2017. PMID: 28864708 Free PMC article. Clinical Trial.
-
Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial.Diabet Med. 2010 Oct;27(10):1159-67. doi: 10.1111/j.1464-5491.2010.03079.x. Diabet Med. 2010. PMID: 20854384 Clinical Trial.
-
Do Cinnamon Supplements Have a Role in Glycemic Control in Type 2 Diabetes? A Narrative Review.J Acad Nutr Diet. 2016 Nov;116(11):1794-1802. doi: 10.1016/j.jand.2016.07.015. Epub 2016 Sep 8. J Acad Nutr Diet. 2016. PMID: 27618575 Free PMC article. Review.
-
To what extent does cinnamon administration improve the glycemic and lipid profiles?Clin Nutr ESPEN. 2018 Oct;27:1-9. doi: 10.1016/j.clnesp.2018.07.011. Epub 2018 Aug 13. Clin Nutr ESPEN. 2018. PMID: 30144878 Review.
Cited by
-
Innovative treatments for obesity and NAFLD: A bibliometric study on antioxidants, herbs, phytochemicals, and natural compounds.Heliyon. 2024 Aug 8;10(16):e35498. doi: 10.1016/j.heliyon.2024.e35498. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220898 Free PMC article. Review.
-
Nutritional Strategies for the Management of Type 2 Diabetes Mellitus: A Narrative Review.Nutrients. 2023 Dec 13;15(24):5096. doi: 10.3390/nu15245096. Nutrients. 2023. PMID: 38140355 Free PMC article. Review.
-
Cinnamic Acid Attenuates Peripheral and Hypothalamic Inflammation in High-Fat Diet-Induced Obese Mice.Pharmaceutics. 2022 Aug 11;14(8):1675. doi: 10.3390/pharmaceutics14081675. Pharmaceutics. 2022. PMID: 36015301 Free PMC article.
-
Exploring the Effects of Energy Constraints on Performance, Body Composition, Endocrinological/Hematological Biomarkers, and Immune System among Athletes: An Overview of the Fasting State.Nutrients. 2022 Aug 4;14(15):3197. doi: 10.3390/nu14153197. Nutrients. 2022. PMID: 35956373 Free PMC article. Review.
-
The Potential Use of Carnosine in Diabetes and Other Afflictions Reported in Long COVID Patients.Front Neurosci. 2022 Jun 22;16:898735. doi: 10.3389/fnins.2022.898735. eCollection 2022. Front Neurosci. 2022. PMID: 35812220 Free PMC article. Review.
References
-
- International Diabetes Federation. IDF Diabetes Atlas, 6th edn. Brussels, Belgium: International Diabetes Federation; http://www.idf.org/diabetesatlas. 2013.
-
- Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34(39):3035–87. 10.1093/eurheartj/eht108 - DOI - PubMed
-
- Garber AJ, Handelsman Y, Einhorn D, Bergman DA, Bloomgarden ZT, Fonseca V, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14(7):933–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

