Embryonic Stem Cell Growth Factors Regulate eIF2α Phosphorylation
- PMID: 26406898
- PMCID: PMC4583406
- DOI: 10.1371/journal.pone.0139076
Embryonic Stem Cell Growth Factors Regulate eIF2α Phosphorylation
Abstract
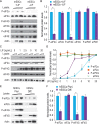
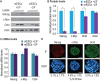
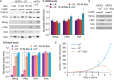
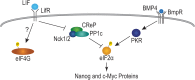
Growth factors and transcription factors are well known to regulate pluripotent stem cells, but less is known about translational control in stem cells. Here, we use embryonic stem cells (ESCs) to investigate a connection between ESC growth factors and eIF2α-mediated translational control (eIF2α phosphorylation promotes protein expression from mRNAs with upstream open-reading frames, or uORFs). We find abundant phosphorylated P-eIF2α (P-eIF2α) in both pluripotent mouse and human ESCs, but little P-eIF2α in ESCs triggered to differentiate. We show that the growth factors LIF (leukemia inhibitory factor) and BMP4 (bone morphogenic protein 4) both maintain P-eIF2α in mESCs, but use distinct mechanisms: LIF inhibits an eIF2α phosphatase whereas BMP4 activates an eIF2α kinase. The mRNAs encoding the pluripotency factors Nanog and c-Myc possess uORFs while Oct4 mRNA does not. We find that salubrinal, a chemical that increases eIF2α phosphorylation, promotes Nanog and c-Myc expression, but not Oct4 expression. These experiments connect ESC growth factors to eIF2α phosphorylation and suggest a chemical substitute for LIF to enhance Nanog and c-Myc expression.
Conflict of interest statement
Figures

Similar articles
-
Impact of eIF2α phosphorylation on the translational landscape of mouse embryonic stem cells.Cell Rep. 2024 Jan 23;43(1):113615. doi: 10.1016/j.celrep.2023.113615. Epub 2023 Dec 29. Cell Rep. 2024. PMID: 38159280 Free PMC article.
-
CHIR99021 enhances Klf4 Expression through β-Catenin Signaling and miR-7a Regulation in J1 Mouse Embryonic Stem Cells.PLoS One. 2016 Mar 3;11(3):e0150936. doi: 10.1371/journal.pone.0150936. eCollection 2016. PLoS One. 2016. PMID: 26938105 Free PMC article.
-
Functional Antagonism between OTX2 and NANOG Specifies a Spectrum of Heterogeneous Identities in Embryonic Stem Cells.Stem Cell Reports. 2017 Nov 14;9(5):1642-1659. doi: 10.1016/j.stemcr.2017.09.019. Epub 2017 Oct 19. Stem Cell Reports. 2017. PMID: 29056334 Free PMC article.
-
A feedback loop comprising PRMT7 and miR-24-2 interplays with Oct4, Nanog, Klf4 and c-Myc to regulate stemness.Nucleic Acids Res. 2016 Dec 15;44(22):10603-10618. doi: 10.1093/nar/gkw788. Epub 2016 Sep 12. Nucleic Acids Res. 2016. PMID: 27625395 Free PMC article.
-
Experimental Evidence Shows Salubrinal, an eIF2α Dephosphorylation Inhibitor, Reduces Xenotoxicant-Induced Cellular Damage.Int J Mol Sci. 2015 Jul 17;16(7):16275-87. doi: 10.3390/ijms160716275. Int J Mol Sci. 2015. PMID: 26193263 Free PMC article. Review.
Cited by
-
Impact of eIF2α phosphorylation on the translational landscape of mouse embryonic stem cells.Cell Rep. 2024 Jan 23;43(1):113615. doi: 10.1016/j.celrep.2023.113615. Epub 2023 Dec 29. Cell Rep. 2024. PMID: 38159280 Free PMC article.
-
Dissolution of ribonucleoprotein condensates by the embryonic stem cell protein L1TD1.Nucleic Acids Res. 2024 Apr 12;52(6):3310-3326. doi: 10.1093/nar/gkad1244. Nucleic Acids Res. 2024. PMID: 38165001 Free PMC article.
-
Translation initiation factors and their relevance in cancer.Curr Opin Genet Dev. 2018 Feb;48:82-88. doi: 10.1016/j.gde.2017.11.001. Epub 2017 Nov 16. Curr Opin Genet Dev. 2018. PMID: 29153484 Free PMC article. Review.
-
Sirtuin 6 is required for the integrated stress response and resistance to inhibition of transcriptional cyclin-dependent kinases.Sci Transl Med. 2023 May 3;15(694):eabn9674. doi: 10.1126/scitranslmed.abn9674. Epub 2023 May 3. Sci Transl Med. 2023. PMID: 37134154 Free PMC article.
-
Translation inhibitory elements from Hoxa3 and Hoxa11 mRNAs use uORFs for translation inhibition.Elife. 2021 Jun 2;10:e66369. doi: 10.7554/eLife.66369. Elife. 2021. PMID: 34076576 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

