Cancer associated fibroblasts have phenotypic and functional characteristics similar to the fibrocytes that represent a novel MDSC subset
- PMID: 26405600
- PMCID: PMC4570137
- DOI: 10.1080/2162402X.2015.1034918
Cancer associated fibroblasts have phenotypic and functional characteristics similar to the fibrocytes that represent a novel MDSC subset
Abstract
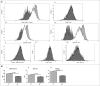
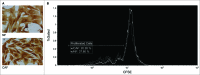
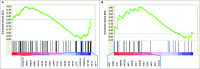
Circulating fibrocytes were reported to represent a novel myeloid-derived suppressor cell (MDSC) subset and they were also proposed to be involved in the tumor immune escape. This novel fibrocyte subset had a surface phenotype resembling non-monocytic MDSCs (CD14-CD11chiCD123-) and exhibited immunomodulatory roles. Most effector functions of fibrocytes (circulating fibroblast-progenitors) are accomplished as tissue fibroblasts, likewise in the tumor microenvironment. Therefore, fibroblasts at tumor tissues should be evaluated whether they display similar molecular/gene expression patterns and functional roles to the blood-borne fibrocytes. A chemically induced rat breast carcinogenesis model was utilized to obtain cancer associated fibroblasts (CAFs). CAFs and normal tissue fibroblasts (NFs) were isolated from cancerous and healthy breast tissues, respectively, using a previously described enzymatic protocol. Both CAFs and NFs were analyzed for cell surface phenotypes by flow cytometry and for gene expression profiles by gene set enrichment analysis (GSEA). PBMCs were cocultured with either NFs or CAFs and proliferations of PBMCs were assessed by CFSE assays. Morphological analyses were performed by immunocytochemistry stainings with vimentin. CAFs were spindle shaped cells unlike their blood-borne counterparts. They did not express CD80 and their MHC-II expression was lower than NFs. Although CAFs expressed the myeloid marker CD11b/c, its expression was lower than that on the circulating fibrocytes. CAFs did not express granulocytic/neutrophilic markers and they seemed to have developed in an environment containing THELPER2-like cytokines. They also showed immunosuppressive effects similar to their blood-borne counterparts. In summary, CAFs showed similar phenotypic and functional characteristics to the circulating fibrocytes that were reported to represent a unique MDSC subset.
Keywords: T cell; breast cancer; chemical carcinogenesis; fibroblast; tumor immunity.
Figures
Similar articles
-
Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer.Blood. 2013 Aug 15;122(7):1105-13. doi: 10.1182/blood-2012-08-449413. Epub 2013 Jun 11. Blood. 2013. PMID: 23757729 Free PMC article.
-
Breast fibroblasts in both cancer and normal tissues induce phenotypic transformation of breast cancer stem cells: a preliminary study.PeerJ. 2018 May 15;6:e4805. doi: 10.7717/peerj.4805. eCollection 2018. PeerJ. 2018. PMID: 29780673 Free PMC article.
-
Activation of Transforming Growth Factor Beta 1 Signaling in Gastric Cancer-associated Fibroblasts Increases Their Motility, via Expression of Rhomboid 5 Homolog 2, and Ability to Induce Invasiveness of Gastric Cancer Cells.Gastroenterology. 2017 Jul;153(1):191-204.e16. doi: 10.1053/j.gastro.2017.03.046. Epub 2017 Apr 5. Gastroenterology. 2017. PMID: 28390866
-
Biological differences between normal and cancer-associated fibroblasts in breast cancer.Heliyon. 2023 Sep 6;9(9):e19803. doi: 10.1016/j.heliyon.2023.e19803. eCollection 2023 Sep. Heliyon. 2023. PMID: 37810030 Free PMC article.
-
Fibrocytes in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1224:79-85. doi: 10.1007/978-3-030-35723-8_6. Adv Exp Med Biol. 2020. PMID: 32036606 Free PMC article. Review.
Cited by
-
Myeloid‑derived suppressor cells: Key immunosuppressive regulators and therapeutic targets in colorectal cancer (Review).Int J Oncol. 2024 Sep;65(3):85. doi: 10.3892/ijo.2024.5673. Epub 2024 Jul 26. Int J Oncol. 2024. PMID: 39054950 Free PMC article. Review.
-
The Cellular Origins of Cancer-Associated Fibroblasts and Their Opposing Contributions to Pancreatic Cancer Growth.Front Cell Dev Biol. 2021 Sep 27;9:743907. doi: 10.3389/fcell.2021.743907. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34646829 Free PMC article. Review.
-
Semaphorin 4D in human head and neck cancer tissue and peripheral blood: A dense fibrotic peri-tumoral stromal phenotype.Oncotarget. 2018 Jan 19;9(13):11126-11144. doi: 10.18632/oncotarget.24277. eCollection 2018 Feb 16. Oncotarget. 2018. PMID: 29541402 Free PMC article.
-
A Comprehensive Pan-Cancer Analysis Identifies CEP55 as a Potential Oncogene and Novel Therapeutic Target.Diagnostics (Basel). 2023 May 2;13(9):1613. doi: 10.3390/diagnostics13091613. Diagnostics (Basel). 2023. PMID: 37175004 Free PMC article.
-
miR200-regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers.Nat Commun. 2018 Mar 13;9(1):1056. doi: 10.1038/s41467-018-03348-z. Nat Commun. 2018. PMID: 29535360 Free PMC article.
References
-
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/10.1038/nri2506 - DOI - PMC - PubMed
-
- Senovilla L, Aranda F, Galluzzi L, Kroemer G. Impact of myeloid cells on the efficacy of anticancer chemotherapy. Curr Opin Immunol 2014; 30C:24-31; PMID:24950501; http://dx.doi.org/10.1016/j.coi.2014.05.009 - DOI - PubMed
-
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181:5791-802; PMID:18832739; http://dx.doi.org/10.4049/jimmunol.181.8.5791 - DOI - PMC - PubMed
-
- Prins RM, Scott GP, Merchant RE, Graf MR. Irradiated tumor cell vaccine for treatment of an established glioma. II. Expansion of myeloid suppressor cells that promote tumor progression. Cancer Immunol, Immunother 2002; 51:190-9; PMID:12012106; http://dx.doi.org/10.1007/s00262-002-0270-x - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
