Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection
- PMID: 26402858
- PMCID: PMC4581628
- DOI: 10.1371/journal.ppat.1005153
Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection
Abstract
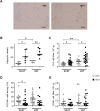
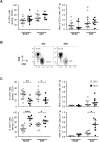
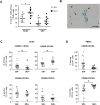
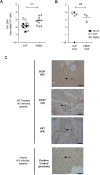
Two of the crucial aspects of human immunodeficiency virus (HIV) infection are (i) viral persistence in reservoirs (precluding viral eradication) and (ii) chronic inflammation (directly associated with all-cause morbidities in antiretroviral therapy (ART)-controlled HIV-infected patients). The objective of the present study was to assess the potential involvement of adipose tissue in these two aspects. Adipose tissue is composed of adipocytes and the stromal vascular fraction (SVF); the latter comprises immune cells such as CD4+ T cells and macrophages (both of which are important target cells for HIV). The inflammatory potential of adipose tissue has been extensively described in the context of obesity. During HIV infection, the inflammatory profile of adipose tissue has been revealed by the occurrence of lipodystrophies (primarily related to ART). Data on the impact of HIV on the SVF (especially in individuals not receiving ART) are scarce. We first analyzed the impact of simian immunodeficiency virus (SIV) infection on abdominal subcutaneous and visceral adipose tissues in SIVmac251 infected macaques and found that both adipocytes and adipose tissue immune cells were affected. The adipocyte density was elevated, and adipose tissue immune cells presented enhanced immune activation and/or inflammatory profiles. We detected cell-associated SIV DNA and RNA in the SVF and in sorted CD4+ T cells and macrophages from adipose tissue. We demonstrated that SVF cells (including CD4+ T cells) are infected in ART-controlled HIV-infected patients. Importantly, the production of HIV RNA was detected by in situ hybridization, and after the in vitro reactivation of sorted CD4+ T cells from adipose tissue. We thus identified adipose tissue as a crucial cofactor in both viral persistence and chronic immune activation/inflammation during HIV infection. These observations open up new therapeutic strategies for limiting the size of the viral reservoir and decreasing low-grade chronic inflammation via the modulation of adipose tissue-related pathways.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Infectious SIV resides in adipose tissue and induces metabolic defects in chronically infected rhesus macaques.Retrovirology. 2016 Apr 27;13:30. doi: 10.1186/s12977-016-0260-2. Retrovirology. 2016. PMID: 27117277 Free PMC article.
-
HIV Persistence in Adipose Tissue Reservoirs.Curr HIV/AIDS Rep. 2018 Feb;15(1):60-71. doi: 10.1007/s11904-018-0378-z. Curr HIV/AIDS Rep. 2018. PMID: 29423731 Free PMC article. Review.
-
Adipose Tissue: Sanctuary for HIV/SIV Persistence and Replication.Trends Microbiol. 2015 Dec;23(12):748-750. doi: 10.1016/j.tim.2015.11.001. Epub 2015 Nov 14. Trends Microbiol. 2015. PMID: 26588868 Free PMC article.
-
Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques.J Virol. 2016 May 27;90(12):5643-5656. doi: 10.1128/JVI.00290-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27030272 Free PMC article.
-
Brain macrophages harbor latent, infectious simian immunodeficiency virus.AIDS. 2019 Dec 1;33 Suppl 2(Suppl 2):S181-S188. doi: 10.1097/QAD.0000000000002269. AIDS. 2019. PMID: 31789817 Free PMC article. Review.
Cited by
-
Does persistent HIV replication explain continued lymphoma incidence in the era of effective antiretroviral therapy?Curr Opin Virol. 2016 Oct;20:71-77. doi: 10.1016/j.coviro.2016.09.001. Epub 2016 Sep 23. Curr Opin Virol. 2016. PMID: 27665065 Free PMC article. Review.
-
Infectious SIV resides in adipose tissue and induces metabolic defects in chronically infected rhesus macaques.Retrovirology. 2016 Apr 27;13:30. doi: 10.1186/s12977-016-0260-2. Retrovirology. 2016. PMID: 27117277 Free PMC article.
-
Adipokines as New Biomarkers of Immune Recovery: Apelin Receptor, RBP4 and ZAG Are Related to CD4+ T-Cell Reconstitution in PLHIV on Suppressive Antiretroviral Therapy.Int J Mol Sci. 2022 Feb 17;23(4):2202. doi: 10.3390/ijms23042202. Int J Mol Sci. 2022. PMID: 35216318 Free PMC article.
-
Non-Alcoholic Fatty Liver Disease in HIV/HBV Patients - a Metabolic Imbalance Aggravated by Antiretroviral Therapy and Perpetuated by the Hepatokine/Adipokine Axis Breakdown.Front Endocrinol (Lausanne). 2022 Mar 9;13:814209. doi: 10.3389/fendo.2022.814209. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35355551 Free PMC article. Review.
-
Specific Biological Features of Adipose Tissue, and Their Impact on HIV Persistence.Front Microbiol. 2019 Dec 17;10:2837. doi: 10.3389/fmicb.2019.02837. eCollection 2019. Front Microbiol. 2019. PMID: 31921023 Free PMC article. Review.
References
-
- Hunt PW, Brenchley J, Sinclair E, Mccune JM, Roland M, Page-shafer K, et al. Relationship between T Cell Activation and CD4+ T Cell Count in HIV-Seropositive Individuals with Undetectable Plasma HIV RNA Levels in the Absence of Therapy. J Infect Dis. 2008;197: 126–133. 10.1086/524143 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

