NIR-driven Smart Theranostic Nanomedicine for On-demand Drug Release and Synergistic Antitumour Therapy
- PMID: 26400780
- PMCID: PMC4585834
- DOI: 10.1038/srep14258
NIR-driven Smart Theranostic Nanomedicine for On-demand Drug Release and Synergistic Antitumour Therapy
Abstract
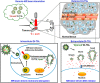
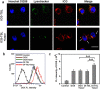
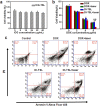
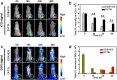
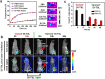
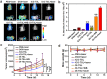
Smart nanoparticles (NPs) that respond to external and internal stimulations have been developing to achieve optimal drug release in tumour. However, applying these smart NPs to attain high antitumour performance is hampered by limited drug carriers and inefficient spatiotemporal control. Here we report a noninvasive NIR-driven, temperature-sensitive DI-TSL (DOX/ICG-loaded temperature sensitive liposomes) co-encapsulating doxorubicin (DOX) and indocyanine green (ICG). This theranostic system applies thermo-responsive lipid to controllably release drug, utilizes the fluorescence (FL) of DOX/ICG to real-time trace the distribution of NPs, and employs DOX/ICG to treat cancer by chemo/photothermal therapy. DI-TSL exhibits uniform size distribution, excellent FL/size stability, enhanced response to NIR-laser, and 3 times increased drug release through laser irradiation. After endocytosis by MCF-7 breast adenocarcinoma cells, DI-TSL in cellular endosomes can cause hyperthermia through laser irradiation, then endosomes are disrupted and DI-TSL 'opens' to release DOX simultaneously for increased cytotoxicity. Furthermore, DI-TSL shows laser-controlled release of DOX in tumour, enhanced ICG and DOX retention by 7 times and 4 times compared with free drugs. Thermo-sensitive DI-TSL manifests high efficiency to promote cell apoptosis, and completely eradicate tumour without side-effect. DI-TSL may provide a smart strategy to release drugs on demand for combinatorial cancer therapy.
Figures

Similar articles
-
Near-infrared light triggered drug delivery system for higher efficacy of combined chemo-photothermal treatment.Acta Biomater. 2017 Mar 15;51:374-392. doi: 10.1016/j.actbio.2016.12.004. Epub 2017 Jan 11. Acta Biomater. 2017. PMID: 28088668
-
Nucleolin-targeted doxorubicin and ICG co-loaded theranostic lipopolymersome for photothermal-chemotherapy of melanoma in vitro and in vivo.Eur J Pharm Biopharm. 2024 Sep;202:114411. doi: 10.1016/j.ejpb.2024.114411. Epub 2024 Jul 14. Eur J Pharm Biopharm. 2024. PMID: 39009192
-
Near-infrared light-sensitive liposomes for the enhanced photothermal tumor treatment by the combination with chemotherapy.Pharm Res. 2014 Mar;31(3):554-65. doi: 10.1007/s11095-013-1180-7. Pharm Res. 2014. PMID: 24022681 Free PMC article.
-
Gold cluster-labeled thermosensitive liposmes enhance triggered drug release in the tumor microenvironment by a photothermal effect.J Control Release. 2015 Oct 28;216:132-9. doi: 10.1016/j.jconrel.2015.08.002. Epub 2015 Aug 4. J Control Release. 2015. PMID: 26247553
-
Current update of a thermosensitive liposomes composed of DPPC and Brij78.J Drug Target. 2018 Jun-Jul;26(5-6):407-419. doi: 10.1080/1061186X.2017.1419361. Epub 2018 Jan 21. J Drug Target. 2018. PMID: 29325469 Review.
Cited by
-
Nanotechnology for Cancer Therapy Based on Chemotherapy.Molecules. 2018 Apr 4;23(4):826. doi: 10.3390/molecules23040826. Molecules. 2018. PMID: 29617302 Free PMC article. Review.
-
Osteotropic Radiolabeled Nanophotosensitizer for Imaging and Treating Multiple Myeloma.ACS Nano. 2020 Apr 28;14(4):4255-4264. doi: 10.1021/acsnano.9b09618. Epub 2020 Apr 6. ACS Nano. 2020. PMID: 32223222 Free PMC article.
-
Emergence and impact of theranostic-nanoformulation of triple therapeutics for combination cancer therapy.Smart Med. 2024 Jan 30;3(1):e20230035. doi: 10.1002/SMMD.20230035. eCollection 2024 Feb. Smart Med. 2024. PMID: 39188518 Free PMC article. Review.
-
Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose.Theranostics. 2018 Jun 7;8(13):3584-3596. doi: 10.7150/thno.25409. eCollection 2018. Theranostics. 2018. PMID: 30026868 Free PMC article.
-
Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems.Pharmaceutics. 2019 Jan 8;11(1):22. doi: 10.3390/pharmaceutics11010022. Pharmaceutics. 2019. PMID: 30625999 Free PMC article. Review.
References
-
- Petros R. A. & DeSimone J. M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug. Discover. 9, 615–627 (2010). - PubMed
-
- Kim H., Lee D., Kim J., Kim T-i. & Kim W. J. Photothermally triggered cytosolic drug delivery via endosome disruption using a functionalized reduced graphene oxide. ACS Nano 7, 6735–6746 (2013). - PubMed
-
- Garcia-Fernandez L. et al. Dual photosensitive polymers with wavelength-selective photoresponse. Adv. Mater. 26, 5012–5017 (2014). - PubMed
-
- Bian T. et al. Spontaneous organization of inorganic nanoparticles into nanovesicles triggered by UV light. Adv. Mater. 26, 5613–5618 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

