The NADPH oxidase Nox4 has anti-atherosclerotic functions
- PMID: 26385958
- PMCID: PMC4751217
- DOI: 10.1093/eurheartj/ehv460
The NADPH oxidase Nox4 has anti-atherosclerotic functions
Abstract
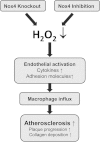
Aims: Oxidative stress is thought to be a risk for cardiovascular disease and NADPH oxidases of the Nox family are important producers of reactive oxygen species. Within the Nox family, the NADPH oxidase Nox4 has a unique position as it is constitutively active and produces H2O2 rather than [Formula: see text] . Nox4 is therefore incapable of scavenging NO and its low constitutive H2O2 production might even be beneficial. We hypothesized that Nox4 acts as an endogenous anti-atherosclerotic enzyme.
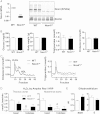
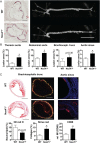
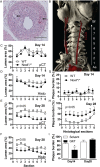
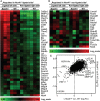
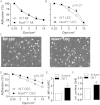
Methods and results: Tamoxifen-induced Nox4-knockout mice were crossed with ApoE⁻/⁻ mice and spontaneous atherosclerosis under regular chow as well as accelerated atherosclerosis in response to partial carotid artery ligation under high-fat diet were determined. Deletion of Nox4 resulted in increased atherosclerosis formation in both models. Mechanistically, pro-atherosclerotic and pro-inflammatory changes in gene expression were observed prior to plaque development. Moreover, inhibition of Nox4 or deletion of the enzyme in the endothelium but not in macrophages resulted in increased adhesion of macrophages to the endothelial surface.
Conclusions: The H2O2-producing NADPH oxidase Nox4 is an endogenous anti-atherosclerotic enzyme. Nox4 inhibitors, currently under clinical evaluation, should be carefully monitored for cardiovascular side-effects.
Keywords: ApoE; Arteriosclerosis; Inflammation; Lipids; NADPH oxidase; Reactive oxygen species; Remodelling.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2015. For permissions please email: journals.permissions@oup.com.
Figures
Comment in
-
Nox4 NADPH oxidase: emerging from the veil of darkness.Eur Heart J. 2015 Dec 21;36(48):3457-9. doi: 10.1093/eurheartj/ehv518. Epub 2015 Oct 1. Eur Heart J. 2015. PMID: 26429798 No abstract available.
Similar articles
-
Reactive Oxygen Species Can Provide Atheroprotection via NOX4-Dependent Inhibition of Inflammation and Vascular Remodeling.Arterioscler Thromb Vasc Biol. 2016 Feb;36(2):295-307. doi: 10.1161/ATVBAHA.115.307012. Epub 2015 Dec 29. Arterioscler Thromb Vasc Biol. 2016. PMID: 26715682
-
Endothelial NADPH oxidase 4 protects ApoE-/- mice from atherosclerotic lesions.Free Radic Biol Med. 2015 Dec;89:1-7. doi: 10.1016/j.freeradbiomed.2015.07.004. Epub 2015 Jul 10. Free Radic Biol Med. 2015. PMID: 26169727 Free PMC article.
-
NADPH oxidase 4 regulates vascular inflammation in aging and atherosclerosis.J Mol Cell Cardiol. 2017 Jan;102:10-21. doi: 10.1016/j.yjmcc.2016.12.004. Epub 2016 Dec 14. J Mol Cell Cardiol. 2017. PMID: 27986445 Free PMC article.
-
Potential benefits and harms of NADPH oxidase type 4 in the kidneys and cardiovascular system.Nephrol Dial Transplant. 2019 Apr 1;34(4):567-576. doi: 10.1093/ndt/gfy161. Nephrol Dial Transplant. 2019. PMID: 29931336 Review.
-
NOX4: a potential therapeutic target for pancreatic cancer and its mechanism.J Transl Med. 2021 Dec 20;19(1):515. doi: 10.1186/s12967-021-03182-w. J Transl Med. 2021. PMID: 34930338 Free PMC article. Review.
Cited by
-
Targeting cardiac fibrosis in heart failure with preserved ejection fraction: mirage or miracle?EMBO Mol Med. 2020 Oct 7;12(10):e10865. doi: 10.15252/emmm.201910865. Epub 2020 Sep 21. EMBO Mol Med. 2020. PMID: 32955172 Free PMC article. Review.
-
PlaqView 2.0: A comprehensive web portal for cardiovascular single-cell genomics.Front Cardiovasc Med. 2022 Aug 8;9:969421. doi: 10.3389/fcvm.2022.969421. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36003902 Free PMC article.
-
Role of ROS and autophagy in the pathological process of atherosclerosis.J Physiol Biochem. 2024 Nov;80(4):743-756. doi: 10.1007/s13105-024-01039-6. Epub 2024 Aug 7. J Physiol Biochem. 2024. PMID: 39110405 Review.
-
Vascular smooth muscle cell phenotypic switching in atherosclerosis.Heliyon. 2024 Sep 10;10(18):e37727. doi: 10.1016/j.heliyon.2024.e37727. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309965 Free PMC article. Review.
-
Role of oxidative stress in calcific aortic valve disease and its therapeutic implications.Cardiovasc Res. 2022 May 6;118(6):1433-1451. doi: 10.1093/cvr/cvab142. Cardiovasc Res. 2022. PMID: 33881501 Free PMC article. Review.
References
-
- Li H, Horke S, Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014;237:208–219. - PubMed
-
- Lonn ME, Dennis JM, Stocker R. Actions of “antioxidants” in the protection against atherosclerosis. Free Radic Biol Med 2012;53:863–884. - PubMed
-
- Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med 2010;49:687–706. - PubMed
-
- Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De MD, Martire B, Pietrogrande MC, Martino S, Gambineri E, Soresina AR, Pignatelli P, Martino F, Basili S, Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation 2009;120:1616–1622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

