Modulating Hippocampal Plasticity with In Vivo Brain Stimulation
- PMID: 26377469
- PMCID: PMC4643097
- DOI: 10.1523/JNEUROSCI.2376-15.2015
Modulating Hippocampal Plasticity with In Vivo Brain Stimulation
Abstract
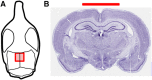
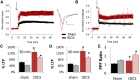
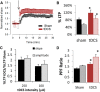
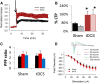
Investigations into the use of transcranial direct current stimulation (tDCS) in relieving symptoms of neurological disorders and enhancing cognitive or motor performance have exhibited promising results. However, the mechanisms by which tDCS effects brain function remain under scrutiny. We have demonstrated that in vivo tDCS in rats produced a lasting effect on hippocampal synaptic plasticity, as measured using extracellular recordings. Ex vivo preparations of hippocampal slices from rats that have been subjected to tDCS of 0.10 or 0.25 mA for 30 min followed by 30 min of recovery time displayed a robust twofold enhancement in long-term potentiation (LTP) induction accompanied by a 30% increase in paired-pulse facilitation (PPF). The magnitude of the LTP effect was greater with 0.25 mA compared with 0.10 mA stimulations, suggesting a dose-dependent relationship between tDCS intensity and its effect on synaptic plasticity. To test the persistence of these observed effects, animals were stimulated in vivo for 30 min at 0.25 mA and then allowed to return to their home cage for 24 h. Observation of the enhanced LTP induction, but not the enhanced PPF, continued 24 h after completion of 0.25 mA of tDCS. Addition of the NMDA blocker AP-5 abolished LTP in both control and stimulated rats but maintained the PPF enhancement in stimulated rats. The observation of enhanced LTP and PPF after tDCS demonstrates that non-invasive electrical stimulation is capable of modifying synaptic plasticity.
Significance statement: Researchers have used brain stimulation such as transcranial direct current stimulation on human subjects to alleviate symptoms of neurological disorders and enhance their performance. Here, using rats, we have investigated the potential mechanisms of how in vivo brain stimulation can produce such effect. We recorded directly on viable brain slices from rats after brain stimulation to detect lasting changes in pattern of neuronal activity. Our results showed that 30 min of brain stimulation in rats induced a robust enhancement in synaptic plasticity, a neuronal process critical for learning and memory. Understanding such molecular effects will lead to a better understanding of the mechanisms by which brain stimulation produces its effects on cognition and performance.
Keywords: brain stimulation; extracellular recording; hippocampus; long term potentiation; rat; tDCS.
Copyright © 2015 the authors 0270-6474/15/3512824-09$15.00/0.
Figures

Similar articles
-
Transcranial direct current stimulation induces hippocampal metaplasticity mediated by brain-derived neurotrophic factor.Neuropharmacology. 2019 Jan;144:358-367. doi: 10.1016/j.neuropharm.2018.11.012. Epub 2018 Nov 12. Neuropharmacology. 2019. PMID: 30439417
-
Polarity and subfield specific effects of transcranial direct current stimulation on hippocampal plasticity.Neurobiol Learn Mem. 2020 Jan;167:107126. doi: 10.1016/j.nlm.2019.107126. Epub 2019 Nov 22. Neurobiol Learn Mem. 2020. PMID: 31765800
-
Developmental regulation of the late phase of long-term potentiation (L-LTP) and metaplasticity in hippocampal area CA1 of the rat.J Neurophysiol. 2012 Feb;107(3):902-12. doi: 10.1152/jn.00780.2011. Epub 2011 Nov 23. J Neurophysiol. 2012. PMID: 22114158 Free PMC article.
-
Animal models of transcranial direct current stimulation: Methods and mechanisms.Clin Neurophysiol. 2016 Nov;127(11):3425-3454. doi: 10.1016/j.clinph.2016.08.016. Epub 2016 Sep 10. Clin Neurophysiol. 2016. PMID: 27693941 Free PMC article. Review.
-
The Physiological Mechanisms of Transcranial Direct Current Stimulation to Enhance Motor Performance: A Narrative Review.Biology (Basel). 2024 Oct 2;13(10):790. doi: 10.3390/biology13100790. Biology (Basel). 2024. PMID: 39452099 Free PMC article. Review.
Cited by
-
Glia: A Neglected Player in Non-invasive Direct Current Brain Stimulation.Front Cell Neurosci. 2016 Aug 8;10:188. doi: 10.3389/fncel.2016.00188. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27551261 Free PMC article.
-
Plasma BDNF Levels Following Transcranial Direct Current Stimulation Allow Prediction of Synaptic Plasticity and Memory Deficits in 3×Tg-AD Mice.Front Cell Dev Biol. 2020 Jul 3;8:541. doi: 10.3389/fcell.2020.00541. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32719795 Free PMC article.
-
Transcranial Direct Current Stimulation to Improve the Dysfunction of Descending Pain Modulatory System Related to Opioids in Chronic Non-cancer Pain: An Integrative Review of Neurobiology and Meta-Analysis.Front Neurosci. 2019 Nov 18;13:1218. doi: 10.3389/fnins.2019.01218. eCollection 2019. Front Neurosci. 2019. PMID: 31803005 Free PMC article.
-
Mechanisms and Effects of Transcranial Direct Current Stimulation.Dose Response. 2017 Feb 9;15(1):1559325816685467. doi: 10.1177/1559325816685467. eCollection 2017 Jan-Mar. Dose Response. 2017. PMID: 28210202 Free PMC article.
-
Optimizing Electrode Montages of Transcranial Direct Current Stimulation for Attentional Bias Modification in Early Abstinent Methamphetamine Users.Front Pharmacol. 2018 Aug 10;9:907. doi: 10.3389/fphar.2018.00907. eCollection 2018. Front Pharmacol. 2018. PMID: 30147655 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
