Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease
- PMID: 26373603
- PMCID: PMC4731417
- DOI: 10.1093/brain/awv262
Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease
Abstract
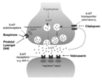
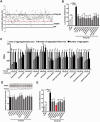
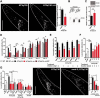
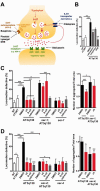
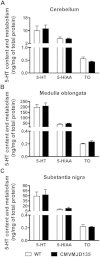
Polyglutamine diseases are a class of dominantly inherited neurodegenerative disorders for which there is no effective treatment. Here we provide evidence that activation of serotonergic signalling is beneficial in animal models of Machado-Joseph disease. We identified citalopram, a selective serotonin reuptake inhibitor, in a small molecule screen of FDA-approved drugs that rescued neuronal dysfunction and reduced aggregation using a Caenorhabditis elegans model of mutant ataxin 3-induced neurotoxicity. MOD-5, the C. elegans orthologue of the serotonin transporter and cellular target of citalopram, and the serotonin receptors SER-1 and SER-4 were strong genetic modifiers of ataxin 3 neurotoxicity and necessary for therapeutic efficacy. Moreover, chronic treatment of CMVMJD135 mice with citalopram significantly reduced ataxin 3 neuronal inclusions and astrogliosis, rescued diminished body weight and strikingly ameliorated motor symptoms. These results suggest that small molecule modulation of serotonergic signalling represents a promising therapeutic target for Machado-Joseph disease.
Keywords: ataxin 3 aggregation; selective serotonin reuptake inhibitor, citalopram; spinocerebellar ataxia type 3; therapy.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Preclinical Evidence Supporting Early Initiation of Citalopram Treatment in Machado-Joseph Disease.Mol Neurobiol. 2019 May;56(5):3626-3637. doi: 10.1007/s12035-018-1332-1. Epub 2018 Sep 1. Mol Neurobiol. 2019. PMID: 30173407
-
Citalopram Reduces Aggregation of ATXN3 in a YAC Transgenic Mouse Model of Machado-Joseph Disease.Mol Neurobiol. 2019 May;56(5):3690-3701. doi: 10.1007/s12035-018-1331-2. Epub 2018 Sep 4. Mol Neurobiol. 2019. PMID: 30187384 Free PMC article.
-
Identification of the 5-HT1A serotonin receptor as a novel therapeutic target in a C. elegans model of Machado-Joseph disease.Neurobiol Dis. 2021 May;152:105278. doi: 10.1016/j.nbd.2021.105278. Epub 2021 Jan 28. Neurobiol Dis. 2021. PMID: 33516872
-
Machado-Joseph Disease: A Stress Combating Deubiquitylating Enzyme Changing Sides.Adv Exp Med Biol. 2020;1233:237-260. doi: 10.1007/978-3-030-38266-7_10. Adv Exp Med Biol. 2020. PMID: 32274760 Review.
-
Mouse models of Machado-Joseph disease and other polyglutamine spinocerebellar ataxias.NeuroRx. 2005 Jul;2(3):480-3. doi: 10.1602/neurorx.2.3.480. NeuroRx. 2005. PMID: 16389311 Free PMC article. Review.
Cited by
-
Organismal Protein Homeostasis Mechanisms.Genetics. 2020 Aug;215(4):889-901. doi: 10.1534/genetics.120.301283. Genetics. 2020. PMID: 32759342 Free PMC article. Review.
-
Preclinical Evidence Supporting Early Initiation of Citalopram Treatment in Machado-Joseph Disease.Mol Neurobiol. 2019 May;56(5):3626-3637. doi: 10.1007/s12035-018-1332-1. Epub 2018 Sep 1. Mol Neurobiol. 2019. PMID: 30173407
-
An Overview of the Current State and the Future of Ataxia Treatments.Neurol Clin. 2020 May;38(2):449-467. doi: 10.1016/j.ncl.2020.01.008. Epub 2020 Feb 27. Neurol Clin. 2020. PMID: 32279720 Free PMC article. Review.
-
Consensus Paper: Strengths and Weaknesses of Animal Models of Spinocerebellar Ataxias and Their Clinical Implications.Cerebellum. 2022 Jun;21(3):452-481. doi: 10.1007/s12311-021-01311-1. Epub 2021 Aug 10. Cerebellum. 2022. PMID: 34378174 Free PMC article.
-
Azepine-Indole Alkaloids From Psychotria nemorosa Modulate 5-HT2A Receptors and Prevent in vivo Protein Toxicity in Transgenic Caenorhabditis elegans.Front Neurosci. 2022 Mar 14;16:826289. doi: 10.3389/fnins.2022.826289. eCollection 2022. Front Neurosci. 2022. PMID: 35360162 Free PMC article.
References
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 2008; 319: 916–9. - PubMed
-
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol 1994; 196: 263–81. - PubMed
-
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology 1999; 21 (2 Suppl): 91S–8S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

