Rho GTPase signalling in cell migration
- PMID: 26363959
- PMCID: PMC4728192
- DOI: 10.1016/j.ceb.2015.08.005
Rho GTPase signalling in cell migration
Abstract
Cells migrate in multiple different ways depending on their environment, which includes the extracellular matrix composition, interactions with other cells, and chemical stimuli. For all types of cell migration, Rho GTPases play a central role, although the relative contribution of each Rho GTPase depends on the environment and cell type. Here, I review recent advances in our understanding of how Rho GTPases contribute to different types of migration, comparing lamellipodium-driven versus bleb-driven migration modes. I also describe how cells migrate across the endothelium. In addition to Rho, Rac and Cdc42, which are well known to regulate migration, I discuss the roles of other less-well characterized members of the Rho family.
Copyright © 2015 The Author. Published by Elsevier Ltd.. All rights reserved.
Figures
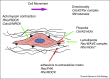

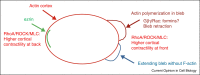
Similar articles
-
Rho GTPases: masters of cell migration.Small GTPases. 2014;5:e29710. doi: 10.4161/sgtp.29710. Epub 2014 Jun 30. Small GTPases. 2014. PMID: 24978113 Free PMC article. Review.
-
Roles of Rho GTPases in leucocyte and leukaemia cell transendothelial migration.Philos Trans R Soc Lond B Biol Sci. 2013 Sep 23;368(1629):20130013. doi: 10.1098/rstb.2013.0013. Print 2013. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24062583 Free PMC article. Review.
-
Rho family GTPases regulate VEGF-stimulated endothelial cell motility.Exp Cell Res. 2001 Sep 10;269(1):73-87. doi: 10.1006/excr.2001.5295. Exp Cell Res. 2001. PMID: 11525641
-
BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study.Mol Cancer. 2011 Sep 23;10:118. doi: 10.1186/1476-4598-10-118. Mol Cancer. 2011. PMID: 21943101 Free PMC article.
-
Roles of Rho-family GTPases in cell polarisation and directional migration.Curr Opin Cell Biol. 2003 Oct;15(5):590-7. doi: 10.1016/s0955-0674(03)00097-8. Curr Opin Cell Biol. 2003. PMID: 14519394 Review.
Cited by
-
Cell Tracking Profiler - a user-driven analysis framework for evaluating 4D live-cell imaging data.J Cell Sci. 2020 Nov 23;133(22):jcs241422. doi: 10.1242/jcs.241422. J Cell Sci. 2020. PMID: 33093241 Free PMC article.
-
Vangl2 promotes the formation of long cytonemes to enable distant Wnt/β-catenin signaling.Nat Commun. 2021 Apr 6;12(1):2058. doi: 10.1038/s41467-021-22393-9. Nat Commun. 2021. PMID: 33824332 Free PMC article.
-
Journey of the mouse primitive endoderm: from specification to maturation.Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210252. doi: 10.1098/rstb.2021.0252. Epub 2022 Oct 17. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36252215 Free PMC article. Review.
-
The Pseudo-Natural Product Rhonin Targets RHOGDI.Angew Chem Int Ed Engl. 2022 Apr 25;61(18):e202115193. doi: 10.1002/anie.202115193. Epub 2022 Mar 2. Angew Chem Int Ed Engl. 2022. PMID: 35170181 Free PMC article.
-
ADP-ribosylation factor-like 4A interacts with Robo1 to promote cell migration by regulating Cdc42 activation.Mol Biol Cell. 2019 Jan 1;30(1):69-81. doi: 10.1091/mbc.E18-01-0001. Epub 2018 Nov 14. Mol Biol Cell. 2019. PMID: 30427759 Free PMC article.
References
-
- Mayor R., Theveneau E. The neural crest. Development. 2013;140:2247–2251. - PubMed
-
- Spano D., Heck C., De Antonellis P., Christofori G., Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012;22:234–249. - PubMed
-
- Friedl P., Locker J., Sahai E., Segall J.E. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14:777–783. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

