Drug and gene delivery across the blood-brain barrier with focused ultrasound
- PMID: 26362698
- PMCID: PMC4656107
- DOI: 10.1016/j.jconrel.2015.08.059
Drug and gene delivery across the blood-brain barrier with focused ultrasound
Abstract
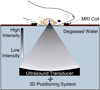
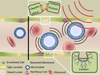
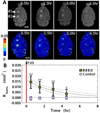
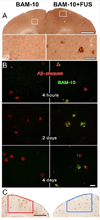
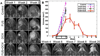
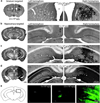
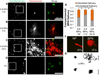
The blood-brain barrier (BBB) remains one of the most significant limitations to treatments of central nervous system (CNS) disorders including brain tumors, neurodegenerative diseases and psychiatric disorders. It is now well-established that focused ultrasound (FUS) in conjunction with contrast agent microbubbles may be used to non-invasively and temporarily disrupt the BBB, allowing localized delivery of systemically administered therapeutic agents as large as 100nm in size to the CNS. Importantly, recent technological advances now permit FUS application through the intact human skull, obviating the need for invasive and risky surgical procedures. When used in combination with magnetic resonance imaging, FUS may be applied precisely to pre-selected CNS targets. Indeed, FUS devices capable of sub-millimeter precision are currently in several clinical trials. FUS mediated BBB disruption has the potential to fundamentally change how CNS diseases are treated, unlocking potential for combinatorial treatments with nanotechnology, markedly increasing the efficacy of existing therapeutics that otherwise do not cross the BBB effectively, and permitting safe repeated treatments. This article comprehensively reviews recent studies on the targeted delivery of therapeutics into the CNS with FUS and offers perspectives on the future of this technology.
Keywords: Blood–brain barrier; CNS drug delivery; Focused ultrasound; Nanoparticles.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound.J Control Release. 2016 Feb 10;223:109-117. doi: 10.1016/j.jconrel.2015.12.034. Epub 2015 Dec 28. J Control Release. 2016. PMID: 26732553 Free PMC article.
-
Focused ultrasound-induced blood-brain barrier opening for non-viral, non-invasive, and targeted gene delivery.J Control Release. 2015 Aug 28;212:1-9. doi: 10.1016/j.jconrel.2015.06.010. Epub 2015 Jun 11. J Control Release. 2015. PMID: 26071631
-
Focused ultrasound-mediated drug delivery through the blood-brain barrier.Expert Rev Neurother. 2015 May;15(5):477-91. doi: 10.1586/14737175.2015.1028369. Expert Rev Neurother. 2015. PMID: 25936845 Free PMC article. Review.
-
Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound.J Control Release. 2014 Sep 10;189:123-132. doi: 10.1016/j.jconrel.2014.06.031. Epub 2014 Jun 28. J Control Release. 2014. PMID: 24979210 Free PMC article.
-
Precision medicine focus on the central nervous system: Non-invasive therapeutic agent delivery with focused ultrasound and microbubbles.Adv Cancer Res. 2024;164:191-240. doi: 10.1016/bs.acr.2024.06.003. Epub 2024 Jul 2. Adv Cancer Res. 2024. PMID: 39306366 Review.
Cited by
-
Protocol to Induce the Temporary Opening of the Blood-Brain Barrier with Short-Time Focused Ultrasound in Rats.Pharmaceutics. 2023 Dec 6;15(12):2733. doi: 10.3390/pharmaceutics15122733. Pharmaceutics. 2023. PMID: 38140074 Free PMC article.
-
Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints.Int J Mol Sci. 2022 Feb 2;23(3):1711. doi: 10.3390/ijms23031711. Int J Mol Sci. 2022. PMID: 35163633 Free PMC article. Review.
-
The new insight into the inflammatory response following focused ultrasound-mediated blood-brain barrier disruption.Fluids Barriers CNS. 2022 Dec 23;19(1):103. doi: 10.1186/s12987-022-00402-3. Fluids Barriers CNS. 2022. PMID: 36564820 Free PMC article.
-
Sonoselective transfection of cerebral vasculature without blood-brain barrier disruption.Proc Natl Acad Sci U S A. 2020 Mar 17;117(11):5644-5654. doi: 10.1073/pnas.1914595117. Epub 2020 Mar 2. Proc Natl Acad Sci U S A. 2020. PMID: 32123081 Free PMC article.
-
Novel Focused Ultrasound Gene Therapy Approach Noninvasively Restores Dopaminergic Neuron Function in a Rat Parkinson's Disease Model.Nano Lett. 2017 Jun 14;17(6):3533-3542. doi: 10.1021/acs.nanolett.7b00616. Epub 2017 May 18. Nano Lett. 2017. PMID: 28511006 Free PMC article.
References
-
- Neurological Disorders: public health challenges
-
- Substance Abuse and Mental Health Services Administration, Results from the 2012 National Survey on Drug Use and Health : Summary of National Findings. 2013 http://store.samhsa.gov/home.
-
- Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289:3135–3144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

