Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling
- PMID: 26350435
- PMCID: PMC5057339
- DOI: 10.1111/cmi.12522
Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling
Abstract
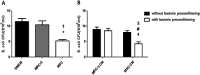
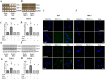
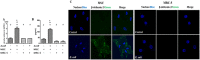
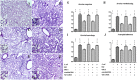
Recently, we demonstrated that intratracheal transplantation of human umbilical cord blood- derived mesenchymal stem cells (MSCs) attenuates Escherichia (E) coli- induced acute lung injury primarily by down- modulating inflammation and enhancing bacterial clearance iQn mice. This study was performed to elucidate the mechanism underlying the antibacterial effects of MSCs. The growth of E. coli in vitro was significantly inhibited only by MSCs or their conditioned medium with bacterial preconditioning, but not by fibroblasts or their conditioned medium. Microarray analysis identified significant up- regulation of toll- like receptors (TLR)- 2 and TLR- 4, and β- defensin 2 (BD2) in MSCs compared with fibroblasts after E. coli exposure. The increased BD2 level and the in vitro antibacterial effects of MSCs were abolished by specific antagonist or by siRNA- mediated knockdown of TLR- 4, but not TLR- 2, and restored by BD2 supplementation. The in vivo down- modulation of the inflammatory response and enhanced bacterial clearance, increased BD2 secretion and the resultant protection against E. coli- induced pneumonia observed only with MSCs, but not fibroblasts, transplantation in mice, were abolished by knockdown of TLR- 4 with siRNA transfection. Our data indicate that BD2 secreted by the MSCs via the TLR- 4 signalling pathway is one of the critical paracrine factors mediating their microbicidal effects against E. coli, both in vitro and in vivo. Furthermore, TLR- 4 from the transplanted MSCs plays a seminal role in attenuating in vivo E. coli- induced pneumonia and the ensuing acute lung injury through both its anti- inflammatory and antibacterial effects.
Keywords: Escherichia coli; acute lung injury; beta- defensin 2; mesenchymal stem cells; toll- like receptor 4.
© 2015 The Authors Cellular Microbiology Published by John Wiley & Sons Ltd.
Figures


Similar articles
-
Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice.Respir Res. 2011 Aug 15;12(1):108. doi: 10.1186/1465-9921-12-108. Respir Res. 2011. PMID: 21843339 Free PMC article.
-
Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia.Thorax. 2012 Jun;67(6):533-9. doi: 10.1136/thoraxjnl-2011-201176. Epub 2012 Jan 16. Thorax. 2012. PMID: 22250097 Free PMC article.
-
Mesenchymal Stromal Cells Primed by Toll-like Receptors 3 and 4 Enhanced Anti-Inflammatory Effects against LPS-Induced Macrophages via Extracellular Vesicles.Int J Mol Sci. 2023 Nov 13;24(22):16264. doi: 10.3390/ijms242216264. Int J Mol Sci. 2023. PMID: 38003458 Free PMC article.
-
Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential.Mediators Inflamm. 2010;2010:865601. doi: 10.1155/2010/865601. Epub 2010 Jun 14. Mediators Inflamm. 2010. PMID: 20628526 Free PMC article. Review.
-
Hematopoietic and Mesenchymal Stromal Cells: New Immunological Roles During Fungal Infections.Stem Cells Dev. 2021 Nov 1;30(21):1049-1055. doi: 10.1089/scd.2021.0211. Epub 2021 Oct 18. Stem Cells Dev. 2021. PMID: 34514808 Review.
Cited by
-
Canine Bone Marrow Mesenchymal Stem Cell Conditioned Media Affect Bacterial Growth, Biofilm-Associated Staphylococcus aureus and AHL-Dependent Quorum Sensing.Microorganisms. 2020 Sep 26;8(10):1478. doi: 10.3390/microorganisms8101478. Microorganisms. 2020. PMID: 32993120 Free PMC article.
-
Enhancement strategies for mesenchymal stem cells and related therapies.Stem Cell Res Ther. 2022 Feb 21;13(1):75. doi: 10.1186/s13287-022-02747-w. Stem Cell Res Ther. 2022. PMID: 35189962 Free PMC article. Review.
-
Potential of Mesenchymal Stem Cell-Derived Exosomes as a Novel Treatment for Female Infertility Caused by Bacterial Infections.Front Microbiol. 2022 Jan 27;12:785649. doi: 10.3389/fmicb.2021.785649. eCollection 2021. Front Microbiol. 2022. PMID: 35154028 Free PMC article. Review.
-
Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Brain Injury in Escherichia coli Meningitis in Newborn Rats.Life (Basel). 2022 Jul 11;12(7):1030. doi: 10.3390/life12071030. Life (Basel). 2022. PMID: 35888118 Free PMC article.
-
Characterization of Angiogenic, Matrix Remodeling, and Antimicrobial Factors in Preterm and Full-Term Human Umbilical Cords.J Dev Biol. 2024 May 1;12(2):13. doi: 10.3390/jdb12020013. J Dev Biol. 2024. PMID: 38804433 Free PMC article.
References
-
- Ahn, S.Y. , Chang, Y.S. , Sung, D.K. , Yoo, H.S. , Sung, S.I. , Choi, S.J. , and Park, W.S. (2015) Cell type‐ dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy 17: 1025–1035. - PubMed
-
- Balan, A. , Lucchini, G. , Schmidt, S. , Schneider, A. , Tramsen, L. , Kuci, S. , et al (2014) Mesenchymal stromal cells in the antimicrobial host response of hematopoietic stem cell recipients with graft‐ versus‐ host disease—friends or foes? Leukemia 28: 1941–1948. - PubMed
-
- Bernardo, M.E. , and Fibbe, W.E. (2013) Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13: 392–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

