A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly
- PMID: 26339030
- PMCID: PMC4727825
- DOI: 10.1126/science.aac7906
A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly
Abstract
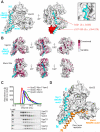
Fusion of intracellular transport vesicles requires soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) and Sec1/Munc18-family (SM) proteins. Membrane-bridging SNARE complexes are critical for fusion, but their spontaneous assembly is inefficient and may require SM proteins in vivo. We report x-ray structures of Vps33, the SM subunit of the yeast homotypic fusion and vacuole protein-sorting (HOPS) complex, bound to two individual SNAREs. The two SNAREs, one from each membrane, are held in the correct orientation and register for subsequent complex assembly. Vps33 and potentially other SM proteins could thus act as templates for generating partially zipped SNARE assembly intermediates. HOPS was essential to mediate SNARE complex assembly at physiological SNARE concentrations. Thus, Vps33 appears to catalyze SNARE complex assembly through specific SNARE motif recognition.
Copyright © 2015, American Association for the Advancement of Science.
Figures


Comment in
-
SEC-uring membrane fusion: a sneak peek at SNARE-complex assembly driven by Sec1-Munc18 proteins.Nat Struct Mol Biol. 2015 Oct;22(10):756-8. doi: 10.1038/nsmb.3094. Nat Struct Mol Biol. 2015. PMID: 26439637 No abstract available.
Similar articles
-
Sec1/Munc18 protein Vps33 binds to SNARE domains and the quaternary SNARE complex.Mol Biol Cell. 2012 Dec;23(23):4611-22. doi: 10.1091/mbc.E12-05-0343. Epub 2012 Oct 10. Mol Biol Cell. 2012. PMID: 23051737 Free PMC article.
-
The Habc domain of the SNARE Vam3 interacts with the HOPS tethering complex to facilitate vacuole fusion.J Biol Chem. 2015 Feb 27;290(9):5405-13. doi: 10.1074/jbc.M114.631465. Epub 2015 Jan 6. J Biol Chem. 2015. PMID: 25564619 Free PMC article.
-
The Sec1/Munc18 protein Vps45 regulates cellular levels of its SNARE binding partners Tlg2 and Snc2 in Saccharomyces cerevisiae.PLoS One. 2012;7(11):e49628. doi: 10.1371/journal.pone.0049628. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23166732 Free PMC article.
-
SNARE complex regulation by phosphorylation.Cell Biochem Biophys. 2006;45(1):111-23. doi: 10.1385/CBB:45:1:111. Cell Biochem Biophys. 2006. PMID: 16679567 Review.
-
Membrane fusion: grappling with SNARE and SM proteins.Science. 2009 Jan 23;323(5913):474-7. doi: 10.1126/science.1161748. Science. 2009. PMID: 19164740 Free PMC article. Review.
Cited by
-
AP-3 vesicle uncoating occurs after HOPS-dependent vacuole tethering.EMBO J. 2020 Oct 15;39(20):e105117. doi: 10.15252/embj.2020105117. Epub 2020 Aug 25. EMBO J. 2020. PMID: 32840906 Free PMC article.
-
Interrogation and validation of the interactome of neuronal Munc18-interacting Mint proteins with AlphaFold2.J Biol Chem. 2024 Jan;300(1):105541. doi: 10.1016/j.jbc.2023.105541. Epub 2023 Dec 9. J Biol Chem. 2024. PMID: 38072052 Free PMC article.
-
Intracellular Vesicle Fusion Requires a Membrane-Destabilizing Peptide Located at the Juxtamembrane Region of the v-SNARE.Cell Rep. 2019 Dec 24;29(13):4583-4592.e3. doi: 10.1016/j.celrep.2019.11.107. Cell Rep. 2019. PMID: 31875562 Free PMC article.
-
Fusion of tethered membranes can be driven by Sec18/NSF and Sec17/αSNAP without HOPS.Elife. 2021 Oct 26;10:e73240. doi: 10.7554/eLife.73240. Elife. 2021. PMID: 34698639 Free PMC article.
-
The road to lysosome-related organelles: Insights from Hermansky-Pudlak syndrome and other rare diseases.Traffic. 2019 Jun;20(6):404-435. doi: 10.1111/tra.12646. Traffic. 2019. PMID: 30945407 Free PMC article. Review.
References
-
- Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Ann. Rev. Cell Dev. Biol. 2012;28:279–308. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

