Isolation of Endoplasmic Reticulum, Mitochondria, and Mitochondria-Associated Membrane and Detergent Resistant Membrane Fractions from Transfected Cells and from Human Cytomegalovirus-Infected Primary Fibroblasts
- PMID: 26331984
- PMCID: PMC4607254
- DOI: 10.1002/0471143030.cb0327s68
Isolation of Endoplasmic Reticulum, Mitochondria, and Mitochondria-Associated Membrane and Detergent Resistant Membrane Fractions from Transfected Cells and from Human Cytomegalovirus-Infected Primary Fibroblasts
Abstract
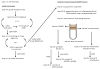
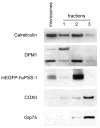
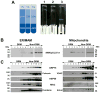
Increasingly mechanistic virology studies require dependable and sensitive methods for isolating purified organelles containing functional cellular sub-domains. The mitochondrial network is, in part, closely apposed to the endoplasmic reticulum (ER). The mitochondria-associated membrane (MAM) fraction provides direct physical contact between the ER and mitochondria. Characterization of the dual localization and trafficking of human cytomegalovirus (HCMV) UL37 proteins required establishing protocols in which the ER and mitochondria could be reliably separated. Because of its documented role in lipid and ceramide transfer from the ER to mitochondria, a method to purify MAM from infected cells was also developed. Two robust procedures were developed to efficiently isolate mitochondria, ER, and MAM fractions while providing substantial protein yields from HCMV-infected primary fibroblasts and from transfected HeLa cells. Furthermore, this unit includes protocols for isolation of detergent resistant membranes from subcellular fractions as well as techniques that allow visualization of the mitochondrial network disruption that occurs in permissively infected cells by their optimal resolution in Percoll gradients.
Keywords: ER; HCMV; MAM; Percoll gradient; differential centrifugation; human fibroblasts; mitochondria; protein localization; subcellular fractionation; sucrose gradient.
Copyright © 2015 John Wiley & Sons, Inc.
Figures




Similar articles
-
Isolation of endoplasmic reticulum, mitochondria, and mitochondria-associated membrane fractions from transfected cells and from human cytomegalovirus-infected primary fibroblasts.Curr Protoc Cell Biol. 2007 Dec;Chapter 3:Unit 3.27. doi: 10.1002/0471143030.cb0327s37. Curr Protoc Cell Biol. 2007. PMID: 18228515
-
Trafficking of UL37 proteins into mitochondrion-associated membranes during permissive human cytomegalovirus infection.J Virol. 2010 Aug;84(15):7898-903. doi: 10.1128/JVI.00885-10. Epub 2010 May 26. J Virol. 2010. PMID: 20504938 Free PMC article.
-
The human cytomegalovirus protein UL37 exon 1 associates with internal lipid rafts.J Virol. 2011 Mar;85(5):2100-11. doi: 10.1128/JVI.01830-10. Epub 2010 Dec 22. J Virol. 2011. PMID: 21177823 Free PMC article.
-
Superresolution imaging of viral protein trafficking.Med Microbiol Immunol. 2015 Jun;204(3):449-60. doi: 10.1007/s00430-015-0395-0. Epub 2015 Feb 28. Med Microbiol Immunol. 2015. PMID: 25724304 Free PMC article. Review.
-
Access of viral proteins to mitochondria via mitochondria-associated membranes.Rev Med Virol. 2009 May;19(3):147-64. doi: 10.1002/rmv.611. Rev Med Virol. 2009. PMID: 19367604 Free PMC article. Review.
Cited by
-
Mitochondrial toxicity induced by a thiourea gold(i) complex: mitochondrial permeability transition and respiratory deficit.Toxicol Res (Camb). 2018 Aug 30;7(6):1081-1090. doi: 10.1039/c8tx00169c. eCollection 2018 Nov 1. Toxicol Res (Camb). 2018. PMID: 30542602 Free PMC article.
-
Cytoplasmic Endonuclease G promotes nonalcoholic fatty liver disease via mTORC2-AKT-ACLY and endoplasmic reticulum stress.Nat Commun. 2023 Oct 4;14(1):6201. doi: 10.1038/s41467-023-41757-x. Nat Commun. 2023. PMID: 37794041 Free PMC article.
-
The interactome of CLUH reveals its association to SPAG5 and its co-translational proximity to mitochondrial proteins.BMC Biol. 2022 Jan 10;20(1):13. doi: 10.1186/s12915-021-01213-y. BMC Biol. 2022. PMID: 35012549 Free PMC article.
-
Superresolution Imaging Identifies That Conventional Trafficking Pathways Are Not Essential for Endoplasmic Reticulum to Outer Mitochondrial Membrane Protein Transport.Sci Rep. 2017 Feb 2;7(1):16. doi: 10.1038/s41598-017-00039-5. Sci Rep. 2017. PMID: 28154412 Free PMC article.
-
Transcriptome-Wide Comparison of Stress Granules and P-Bodies Reveals that Translation Plays a Major Role in RNA Partitioning.Mol Cell Biol. 2019 Nov 25;39(24):e00313-19. doi: 10.1128/MCB.00313-19. Print 2019 Dec 15. Mol Cell Biol. 2019. PMID: 31591142 Free PMC article.
References
-
- Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microscopy research and technique. 1994;27:198–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

