Analysis of Nearly One Thousand Mammalian Mirtrons Reveals Novel Features of Dicer Substrates
- PMID: 26325366
- PMCID: PMC4556696
- DOI: 10.1371/journal.pcbi.1004441
Analysis of Nearly One Thousand Mammalian Mirtrons Reveals Novel Features of Dicer Substrates
Abstract
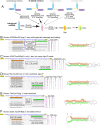
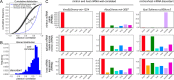
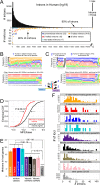
Mirtrons are microRNA (miRNA) substrates that utilize the splicing machinery to bypass the necessity of Drosha cleavage for their biogenesis. Expanding our recent efforts for mammalian mirtron annotation, we use meta-analysis of aggregate datasets to identify ~500 novel mouse and human introns that confidently generate diced small RNA duplexes. These comprise nearly 1000 total loci distributed in four splicing-mediated biogenesis subclasses, with 5'-tailed mirtrons as, by far, the dominant subtype. Thus, mirtrons surprisingly comprise a substantial fraction of endogenous Dicer substrates in mammalian genomes. Although mirtron-derived small RNAs exhibit overall expression correlation with their host mRNAs, we observe a subset with substantial differences that suggest regulated processing or accumulation. We identify characteristic sequence, length, and structural features of mirtron loci that distinguish them from bulk introns, and find that mirtrons preferentially emerge from genes with larger numbers of introns. While mirtrons generate miRNA-class regulatory RNAs, we also find that mirtrons exhibit many features that distinguish them from canonical miRNAs. We observe that conventional mirtron hairpins are substantially longer than Drosha-generated pre-miRNAs, indicating that the characteristic length of canonical pre-miRNAs is not a general feature of Dicer substrate hairpins. In addition, mammalian mirtrons exhibit unique patterns of ordered 5' and 3' heterogeneity, which reveal hidden complexity in miRNA processing pathways. These include broad 3'-uridylation of mirtron hairpins, atypically heterogeneous 5' termini that may result from exonucleolytic processing, and occasionally robust decapitation of the 5' guanine (G) of mirtron-5p species defined by splicing. Altogether, this study reveals that this extensive class of non-canonical miRNA bears a multitude of characteristic properties, many of which raise general mechanistic questions regarding the processing of endogenous hairpin transcripts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Comparing miRNA structure of mirtrons and non-mirtrons.BMC Genomics. 2018 Feb 9;19(Suppl 3):114. doi: 10.1186/s12864-018-4473-8. BMC Genomics. 2018. PMID: 29504892 Free PMC article.
-
Promiscuous splicing-derived hairpins are dominant substrates of tailing-mediated defense of miRNA biogenesis in mammals.Cell Rep. 2023 Feb 28;42(2):112111. doi: 10.1016/j.celrep.2023.112111. Epub 2023 Feb 16. Cell Rep. 2023. PMID: 36800291 Free PMC article.
-
The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila.Cell. 2007 Jul 13;130(1):89-100. doi: 10.1016/j.cell.2007.06.028. Epub 2007 Jun 28. Cell. 2007. PMID: 17599402 Free PMC article.
-
Mirtrons: microRNA biogenesis via splicing.Biochimie. 2011 Nov;93(11):1897-904. doi: 10.1016/j.biochi.2011.06.017. Epub 2011 Jun 21. Biochimie. 2011. PMID: 21712066 Free PMC article. Review.
-
Biogenesis, characterization, and functions of mirtrons.Wiley Interdiscip Rev RNA. 2022 Jan;13(1):e1680. doi: 10.1002/wrna.1680. Epub 2021 Jun 21. Wiley Interdiscip Rev RNA. 2022. PMID: 34155810 Review.
Cited by
-
Small RNA sequences derived from pre-microRNAs in the supraspliceosome.Nucleic Acids Res. 2018 Nov 16;46(20):11014-11029. doi: 10.1093/nar/gky791. Nucleic Acids Res. 2018. PMID: 30203035 Free PMC article.
-
Distinguishing mirtrons from canonical miRNAs with data exploration and machine learning methods.Sci Rep. 2018 May 15;8(1):7560. doi: 10.1038/s41598-018-25578-3. Sci Rep. 2018. PMID: 29765080 Free PMC article.
-
MicroRNAs and Stem-like Properties: The Complex Regulation Underlying Stemness Maintenance and Cancer Development.Biomolecules. 2021 Jul 21;11(8):1074. doi: 10.3390/biom11081074. Biomolecules. 2021. PMID: 34439740 Free PMC article. Review.
-
Knockdown and replacement therapy mediated by artificial mirtrons in spinocerebellar ataxia 7.Nucleic Acids Res. 2017 Jul 27;45(13):7870-7885. doi: 10.1093/nar/gkx483. Nucleic Acids Res. 2017. PMID: 28575281 Free PMC article.
-
MicroRNAs Regulating Mitochondrial Function in Cardiac Diseases.Front Pharmacol. 2021 May 28;12:663322. doi: 10.3389/fphar.2021.663322. eCollection 2021. Front Pharmacol. 2021. PMID: 34122082 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

