Proteoliposomes with the ability to transport Ca(2+) into the vesicles and hydrolyze phosphosubstrates on their surface
- PMID: 26325078
- PMCID: PMC5257277
- DOI: 10.1016/j.abb.2015.08.018
Proteoliposomes with the ability to transport Ca(2+) into the vesicles and hydrolyze phosphosubstrates on their surface
Abstract
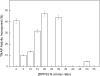
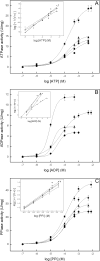
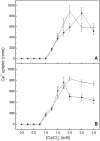
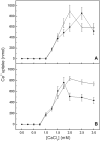
We describe the production of stable DPPC and DPPC:DPPS-proteoliposomes harboring annexin V (AnxA5) and tissue-nonspecific alkaline phosphatase (TNAP) and their use to investigate whether the presence of AnxA5 impacts the kinetic parameters for hydrolysis of TNAP substrates at physiological pH. The best catalytic efficiency was achieved in DPPS 10%-proteoliposomes (molar ratio), conditions that also increased the specificity of TNAP hydrolysis of PPi. Melting behavior of liposomes and proteoliposomes was analyzed via differential scanning calorimetry. The presence of 10% DPPS in DPPC-liposomes causes a broadening of the transition peaks, with AnxA5 and TNAP promoting a decrease in ΔH values. AnxA5 was able to mediate Ca(2+)-influx into the DPPC and DPPC:DPPS 10%-vesicles at physiological Ca(2+) concentrations (∼2 mM). This process was not affected by the presence of TNAP in the proteoliposomes. However, AnxA5 significantly affects the hydrolysis of TNAP substrates. Studies with GUVs confirmed the functional reconstitution of AnxA5 in the mimetic systems. These proteoliposomes are useful as mimetics of mineralizing cell-derived matrix vesicles, known to be responsible for the initiation of endochondral ossification, as they successfully transport Ca(2+) and possess the ability to hydrolyze phosphosubstrates in the lipid-water interface.
Keywords: Alkaline phosphatase; Annexin V; Calorimetry; DPPC; DPPS; Liposome; Matrix vesicles; Proteoliposome.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Topographic analysis by atomic force microscopy of proteoliposomes matrix vesicle mimetics harboring TNAP and AnxA5.Biochim Biophys Acta Biomembr. 2017 Oct;1859(10):1911-1920. doi: 10.1016/j.bbamem.2017.05.010. Epub 2017 May 23. Biochim Biophys Acta Biomembr. 2017. PMID: 28549727 Free PMC article.
-
Matrix vesicle biomimetics harboring Annexin A5 and alkaline phosphatase bind to the native collagen matrix produced by mineralizing vascular smooth muscle cells.Biochim Biophys Acta Gen Subj. 2020 Aug;1864(8):129629. doi: 10.1016/j.bbagen.2020.129629. Epub 2020 Apr 29. Biochim Biophys Acta Gen Subj. 2020. PMID: 32360152 Free PMC article.
-
The effect of cholesterol on the reconstitution of alkaline phosphatase into liposomes.Biophys Chem. 2010 Nov;152(1-3):74-9. doi: 10.1016/j.bpc.2010.08.002. Epub 2010 Aug 14. Biophys Chem. 2010. PMID: 20810204
-
Past, present, and future of annexin A5: from protein discovery to clinical applications.J Nucl Med. 2005 Dec;46(12):2035-50. J Nucl Med. 2005. PMID: 16330568 Review.
-
Biophysical aspects of biomineralization.Biophys Rev. 2017 Oct;9(5):747-760. doi: 10.1007/s12551-017-0315-1. Epub 2017 Aug 29. Biophys Rev. 2017. PMID: 28852989 Free PMC article. Review.
Cited by
-
Annexins A2, A6 and Fetuin-A Affect the Process of Mineralization in Vesicles Derived from Human Osteoblastic hFOB 1.19 and Osteosarcoma Saos-2 Cells.Int J Mol Sci. 2021 Apr 13;22(8):3993. doi: 10.3390/ijms22083993. Int J Mol Sci. 2021. PMID: 33924370 Free PMC article.
-
Phosphatidylserine controls calcium phosphate nucleation and growth on lipid monolayers: A physicochemical understanding of matrix vesicle-driven biomineralization.J Struct Biol. 2020 Nov 1;212(2):107607. doi: 10.1016/j.jsb.2020.107607. Epub 2020 Aug 26. J Struct Biol. 2020. PMID: 32858148 Free PMC article.
-
Ultrasensitive Diamond Microelectrode Application in the Detection of Ca2+ Transport by AnnexinA5-Containing Nanostructured Liposomes.Biosensors (Basel). 2022 Jul 14;12(7):525. doi: 10.3390/bios12070525. Biosensors (Basel). 2022. PMID: 35884328 Free PMC article.
-
Localization of Annexin A6 in Matrix Vesicles During Physiological Mineralization.Int J Mol Sci. 2020 Feb 18;21(4):1367. doi: 10.3390/ijms21041367. Int J Mol Sci. 2020. PMID: 32085611 Free PMC article.
-
Shedding Light on the Role of Na,K-ATPase as a Phosphatase during Matrix-Vesicle-Mediated Mineralization.Int J Mol Sci. 2022 Dec 1;23(23):15072. doi: 10.3390/ijms232315072. Int J Mol Sci. 2022. PMID: 36499456 Free PMC article.
References
-
- Wuthier RE. Fed Proc. 1976;35:117–121. - PubMed
-
- Anderson HC. Scan Electron Microsc Pt. 1984;2:953–964. - PubMed
-
- Anderson HC. Curr Rheumatol Rep. 2003;5:222–226. - PubMed
-
- Thouverey C, Strzelecka-Kiliszek A, Balcerzak M, Buchet R, Pikula SJ. Cel Biochem. 2009;106:127–138. - PubMed
-
- Buchet R, Pikula S, Magne D, Mebarek S. Methods Mol Biol. 2013;1053:115–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

