Receptor sequestration in response to β-arrestin-2 phosphorylation by ERK1/2 governs steady-state levels of GPCR cell-surface expression
- PMID: 26324936
- PMCID: PMC4577186
- DOI: 10.1073/pnas.1508836112
Receptor sequestration in response to β-arrestin-2 phosphorylation by ERK1/2 governs steady-state levels of GPCR cell-surface expression
Abstract
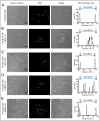
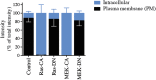
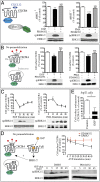
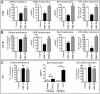
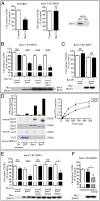
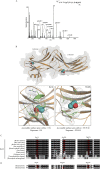
MAPKs are activated in response to G protein-coupled receptor (GPCR) stimulation and play essential roles in regulating cellular processes downstream of these receptors. However, very little is known about the reciprocal effect of MAPK activation on GPCRs. To investigate possible crosstalk between the MAPK and GPCRs, we assessed the effect of ERK1/2 on the activity of several GPCR family members. We found that ERK1/2 activation leads to a reduction in the steady-state cell-surface expression of many GPCRs because of their intracellular sequestration. This subcellular redistribution resulted in a global dampening of cell responsiveness, as illustrated by reduced ligand-mediated G-protein activation and second-messenger generation as well as blunted GPCR kinases and β-arrestin recruitment. This ERK1/2-mediated regulatory process was observed for GPCRs that can interact with β-arrestins, such as type-2 vasopressin, type-1 angiotensin, and CXC type-4 chemokine receptors, but not for the prostaglandin F receptor that cannot interact with β-arrestin, implicating this scaffolding protein in the receptor's subcellular redistribution. Complementation experiments in mouse embryonic fibroblasts lacking β-arrestins combined with in vitro kinase assays revealed that β-arrestin-2 phosphorylation on Ser14 and Thr276 is essential for the ERK1/2-promoted GPCR sequestration. This previously unidentified regulatory mechanism was observed after constitutive activation as well as after receptor tyrosine kinase- or GPCR-mediated activation of ERK1/2, suggesting that it is a central node in the tonic regulation of cell responsiveness to GPCR stimulation, acting both as an effector and a negative regulator.
Keywords: G protein-coupled receptor; MAPK; cell signaling; internalization; β-arrestin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

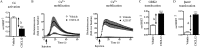


Similar articles
-
The three α1-adrenoceptor subtypes show different spatio-temporal mechanisms of internalization and ERK1/2 phosphorylation.Biochim Biophys Acta. 2013 Oct;1833(10):2322-33. doi: 10.1016/j.bbamcr.2013.06.013. Epub 2013 Jun 21. Biochim Biophys Acta. 2013. PMID: 23797059
-
Phosphorylation of β-arrestin2 at Thr383 by MEK underlies β-arrestin-dependent activation of Erk1/2 by GPCRs.Elife. 2017 Feb 7;6:e23777. doi: 10.7554/eLife.23777. Elife. 2017. PMID: 28169830 Free PMC article.
-
Activation and nuclear translocation of ERK1/2 by the formyl peptide receptor is regulated by G protein and is not dependent on beta-arrestin translocation or receptor endocytosis.Cell Signal. 2005 Oct;17(10):1300-11. doi: 10.1016/j.cellsig.2005.01.006. Epub 2005 Feb 23. Cell Signal. 2005. PMID: 16038804
-
Beta-arrestin signaling and regulation of transcription.J Cell Sci. 2007 Jan 15;120(Pt 2):213-8. doi: 10.1242/jcs.03338. J Cell Sci. 2007. PMID: 17215450 Review.
-
Dissecting the signaling features of the multi-protein complex GPCR/β-arrestin/ERK1/2.Eur J Cell Biol. 2018 Jun;97(5):349-358. doi: 10.1016/j.ejcb.2018.04.001. Epub 2018 Apr 4. Eur J Cell Biol. 2018. PMID: 29665971 Review.
Cited by
-
The RanBP2/RanGAP1-SUMO complex gates β-arrestin2 nuclear entry to regulate the Mdm2-p53 signaling axis.Oncogene. 2021 Mar;40(12):2243-2257. doi: 10.1038/s41388-021-01704-w. Epub 2021 Mar 1. Oncogene. 2021. PMID: 33649538
-
Heterologous phosphorylation-induced formation of a stability lock permits regulation of inactive receptors by β-arrestins.J Biol Chem. 2018 Jan 19;293(3):876-892. doi: 10.1074/jbc.M117.813139. Epub 2017 Nov 16. J Biol Chem. 2018. PMID: 29146594 Free PMC article.
-
Differential phosphorylation signals control endocytosis of GPR15.Mol Biol Cell. 2017 Aug 15;28(17):2267-2281. doi: 10.1091/mbc.E16-09-0627. Epub 2017 Jun 14. Mol Biol Cell. 2017. PMID: 28615320 Free PMC article.
-
Modulation of polycystic kidney disease by G-protein coupled receptors and cyclic AMP signaling.Cell Signal. 2020 Aug;72:109649. doi: 10.1016/j.cellsig.2020.109649. Epub 2020 Apr 23. Cell Signal. 2020. PMID: 32335259 Free PMC article. Review.
-
Discovery of a dual Ras and ARF6 inhibitor from a GPCR endocytosis screen.Nat Commun. 2021 Aug 3;12(1):4688. doi: 10.1038/s41467-021-24968-y. Nat Commun. 2021. PMID: 34344896 Free PMC article.
References
-
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726–735. - PubMed
-
- Roskoski R., Jr ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol Res. 2012;66(2):105–143. - PubMed
-
- Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110(6):669–672. - PubMed
-
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26(22):3113–3121. - PubMed
-
- Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: Emerging paradigms. Trends Pharmacol Sci. 2001;22(7):368–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

