Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism
- PMID: 26324905
- PMCID: PMC4586853
- DOI: 10.1073/pnas.1514475112
Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism
Erratum in
-
Correction for Prusiner et al., Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism.Proc Natl Acad Sci U S A. 2024 Jun 11;121(24):e2408899121. doi: 10.1073/pnas.2408899121. Epub 2024 Jun 3. Proc Natl Acad Sci U S A. 2024. PMID: 38830111 Free PMC article. No abstract available.
Abstract
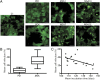
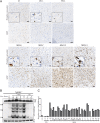
Prions are proteins that adopt alternative conformations that become self-propagating; the PrP(Sc) prion causes the rare human disorder Creutzfeldt-Jakob disease (CJD). We report here that multiple system atrophy (MSA) is caused by a different human prion composed of the α-synuclein protein. MSA is a slowly evolving disorder characterized by progressive loss of autonomic nervous system function and often signs of parkinsonism; the neuropathological hallmark of MSA is glial cytoplasmic inclusions consisting of filaments of α-synuclein. To determine whether human α-synuclein forms prions, we examined 14 human brain homogenates for transmission to cultured human embryonic kidney (HEK) cells expressing full-length, mutant human α-synuclein fused to yellow fluorescent protein (α-syn140*A53T-YFP) and TgM83(+/-) mice expressing α-synuclein (A53T). The TgM83(+/-) mice that were hemizygous for the mutant transgene did not develop spontaneous illness; in contrast, the TgM83(+/+) mice that were homozygous developed neurological dysfunction. Brain extracts from 14 MSA cases all transmitted neurodegeneration to TgM83(+/-) mice after incubation periods of ∼120 d, which was accompanied by deposition of α-synuclein within neuronal cell bodies and axons. All of the MSA extracts also induced aggregation of α-syn*A53T-YFP in cultured cells, whereas none of six Parkinson's disease (PD) extracts or a control sample did so. Our findings argue that MSA is caused by a unique strain of α-synuclein prions, which is different from the putative prions causing PD and from those causing spontaneous neurodegeneration in TgM83(+/+) mice. Remarkably, α-synuclein is the first new human prion to be identified, to our knowledge, since the discovery a half century ago that CJD was transmissible.
Keywords: Parkinson's disease; neurodegeneration; strains; synucleinopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Expanding the prion disease repertoire.Proc Natl Acad Sci U S A. 2015 Sep 22;112(38):11748-9. doi: 10.1073/pnas.1515143112. Epub 2015 Sep 1. Proc Natl Acad Sci U S A. 2015. PMID: 26330608 Free PMC article. No abstract available.
-
Is Multiple System Atrophy a New Prion Disorder?Mov Disord. 2016 Mar;31(3):300. doi: 10.1002/mds.26537. Epub 2016 Feb 1. Mov Disord. 2016. PMID: 26833797 No abstract available.
Similar articles
-
Transmission of multiple system atrophy prions to transgenic mice.Proc Natl Acad Sci U S A. 2013 Nov 26;110(48):19555-60. doi: 10.1073/pnas.1318268110. Epub 2013 Nov 11. Proc Natl Acad Sci U S A. 2013. PMID: 24218576 Free PMC article.
-
Multiple system atrophy prions retain strain specificity after serial propagation in two different Tg(SNCA*A53T) mouse lines.Acta Neuropathol. 2019 Mar;137(3):437-454. doi: 10.1007/s00401-019-01959-4. Epub 2019 Jan 28. Acta Neuropathol. 2019. PMID: 30690664 Free PMC article.
-
MSA prions exhibit remarkable stability and resistance to inactivation.Acta Neuropathol. 2018 Jan;135(1):49-63. doi: 10.1007/s00401-017-1762-2. Epub 2017 Aug 28. Acta Neuropathol. 2018. PMID: 28849371 Free PMC article.
-
Is Multiple System Atrophy a Prion-like Disorder?Int J Mol Sci. 2021 Sep 18;22(18):10093. doi: 10.3390/ijms221810093. Int J Mol Sci. 2021. PMID: 34576255 Free PMC article. Review.
-
ɑ-Synuclein strains and the variable pathologies of synucleinopathies.J Neurochem. 2016 Oct;139 Suppl 1:256-274. doi: 10.1111/jnc.13595. Epub 2016 Mar 30. J Neurochem. 2016. PMID: 26924014 Review.
Cited by
-
In Search of Effective Treatments Targeting α-Synuclein Toxicity in Synucleinopathies: Pros and Cons.Front Cell Dev Biol. 2020 Sep 4;8:559791. doi: 10.3389/fcell.2020.559791. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015057 Free PMC article. Review.
-
Interplay of Ferritin Accumulation and Ferroportin Loss in Ageing Brain: Implication for Protein Aggregation in Down Syndrome Dementia, Alzheimer's, and Parkinson's Diseases.Int J Mol Sci. 2022 Jan 19;23(3):1060. doi: 10.3390/ijms23031060. Int J Mol Sci. 2022. PMID: 35162984 Free PMC article.
-
Autoclave treatment fails to completely inactivate DLB alpha-synuclein seeding activity.Biochem Biophys Rep. 2023 Mar 3;34:101446. doi: 10.1016/j.bbrep.2023.101446. eCollection 2023 Jul. Biochem Biophys Rep. 2023. PMID: 36923008 Free PMC article.
-
Parkinson's disease and multiple system atrophy patient iPSC-derived oligodendrocytes exhibit alpha-synuclein-induced changes in maturation and immune reactive properties.Proc Natl Acad Sci U S A. 2022 Mar 22;119(12):e2111405119. doi: 10.1073/pnas.2111405119. Epub 2022 Mar 16. Proc Natl Acad Sci U S A. 2022. PMID: 35294277 Free PMC article.
-
Spreading of Alpha Synuclein from Glioblastoma Cells towards Astrocytes Correlates with Stem-like Properties.Cancers (Basel). 2022 Mar 10;14(6):1417. doi: 10.3390/cancers14061417. Cancers (Basel). 2022. PMID: 35326570 Free PMC article.
References
-
- Gajdusek DC, Gibbs CJ, Jr, Alpers M. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature. 1966;209(5025):794–796. - PubMed
-
- Gibbs CJ, Jr, Gajdusek DC. An update on long-term in vivo and in vitro studies designed to identify a virus as the cause of amyotrophic lateral sclerosis, parkinsonism dementia, and Parkinson’s disease. Adv Neurol. 1982;36:343–353. - PubMed
-
- Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94(1-3):79–100. - PubMed
-
- Spillantini MG, et al. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251(3):205–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG002132/AG/NIA NIH HHS/United States
- AG005134/AG/NIA NIH HHS/United States
- P01 AG002132/AG/NIA NIH HHS/United States
- R37 AG031220/AG/NIA NIH HHS/United States
- P01 AG010770/AG/NIA NIH HHS/United States
- G-0909/PUK_/Parkinson's UK/United Kingdom
- P01 AG021601/AG/NIA NIH HHS/United States
- AG021601/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- AG031220/AG/NIA NIH HHS/United States
- AG010770/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

