Interleukin-25 Mediates Transcriptional Control of PD-L1 via STAT3 in Multipotent Human Mesenchymal Stromal Cells (hMSCs) to Suppress Th17 Responses
- PMID: 26321145
- PMCID: PMC4618596
- DOI: 10.1016/j.stemcr.2015.07.013
Interleukin-25 Mediates Transcriptional Control of PD-L1 via STAT3 in Multipotent Human Mesenchymal Stromal Cells (hMSCs) to Suppress Th17 Responses
Abstract
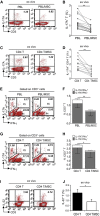
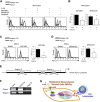
Multipotent human mesenchymal stromal cells (hMSCs) harbor immunomodulatory properties that are therapeutically relevant. One of the most clinically important populations of leukocytes is the interleukin-17A (IL-17A)-secreting T (Th17) lymphocytes. However, mechanisms of hMSC and Th17 cell interactions are incompletely resolved. We found that, along with Th1 responses, hMSCs strongly suppressed Th17 responses and this required both IL-25--also known as IL--17E-as well as programmed death ligand-1 (PD-L1), a potent cell surface ligand for tolerance induction. Knockdown of IL-25 expression in hMSCs abrogated Th17 suppression in vitro and in vivo. However, IL-25 alone was insufficient to significantly suppress Th17 responses, which also required surface PD-L1 expression. Critically, IL-25 upregulated PD-L1 surface expression through the signaling pathways of JNK and STAT3, with STAT3 found to constitutively occupy the proximal region of the PD-L1 promoter. Our findings demonstrate the complexities of hMSC-mediated Th17 suppression, and highlight the IL-25/STAT3/PD-L1 axis as a candidate therapeutic target.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Conversion of Th17 into IL-17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy.J Immunol. 2014 Nov 15;193(10):4988-99. doi: 10.4049/jimmunol.1401776. Epub 2014 Oct 10. J Immunol. 2014. PMID: 25305313
-
Different roles of PD-L1 and FasL in immunomodulation mediated by human placenta-derived mesenchymal stem cells.Hum Immunol. 2013 Mar;74(3):267-76. doi: 10.1016/j.humimm.2012.12.011. Epub 2012 Dec 20. Hum Immunol. 2013. PMID: 23261407
-
[PD-L1/PD-L2 on human placenta-derived mesenchymal stem cells inhibits the IL-17 secretion of peripheral blood T cells].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013 Feb;29(2):132-6. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013. PMID: 23388330 Chinese.
-
Expression of Th17-related genes in PHA/IL-2-activated human T cells by Fas signaling via caspase-1- and Stat3-dependent pathway.Cell Immunol. 2013 Feb;281(2):101-10. doi: 10.1016/j.cellimm.2013.03.002. Epub 2013 Mar 28. Cell Immunol. 2013. PMID: 23590971
-
IL-6, IL-17 and STAT3: a holy trinity in auto-immunity?Front Biosci (Landmark Ed). 2012 Jun 1;17(6):2306-26. doi: 10.2741/4054. Front Biosci (Landmark Ed). 2012. PMID: 22652781 Review.
Cited by
-
Enhanced Immunomodulation in Inflammatory Environments Favors Human Cardiac Mesenchymal Stromal-Like Cells for Allogeneic Cell Therapies.Front Immunol. 2019 Jul 23;10:1716. doi: 10.3389/fimmu.2019.01716. eCollection 2019. Front Immunol. 2019. PMID: 31396228 Free PMC article.
-
Interleukin 25 and its biological features and function in intestinal diseases.Cent Eur J Immunol. 2022;47(4):362-372. doi: 10.5114/ceji.2022.124416. Epub 2023 Jan 31. Cent Eur J Immunol. 2022. PMID: 36817397 Free PMC article. Review.
-
Targeting the PD-L1/DNMT1 axis in acquired resistance to sorafenib in human hepatocellular carcinoma.Oncol Rep. 2017 Aug;38(2):899-907. doi: 10.3892/or.2017.5722. Epub 2017 Jun 14. Oncol Rep. 2017. PMID: 28627705 Free PMC article.
-
Immune checkpoint dysfunction in large and medium vessel vasculitis.Am J Physiol Heart Circ Physiol. 2017 May 1;312(5):H1052-H1059. doi: 10.1152/ajpheart.00024.2017. Epub 2017 Mar 17. Am J Physiol Heart Circ Physiol. 2017. PMID: 28314758 Free PMC article. Review.
-
Resident vs nonresident multipotent mesenchymal stromal cell interactions with B lymphocytes result in disparate outcomes.Stem Cells Transl Med. 2021 May;10(5):711-724. doi: 10.1002/sctm.20-0289. Epub 2021 Jan 28. Stem Cells Transl Med. 2021. PMID: 33506633 Free PMC article.
References
-
- Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. - PubMed
-
- Aksu A.E., Horibe E., Sacks J., Ikeguchi R., Breitinger J., Scozio M., Unadkat J., Feili-Hariri M. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin. Immunol. 2008;127:348–358. - PubMed
-
- Antonysamy M.A., Fanslow W.C., Fu F., Li W., Qian S., Troutt A.B., Thomson A.W. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J. Immunol. 1999;162:577–584. - PubMed
-
- Bartholomew A., Sturgeon C., Siatskas M., Ferrer K., McIntosh K., Patil S., Hardy W., Devine S., Ucker D., Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30:42–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

