MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation
- PMID: 26320658
- PMCID: PMC4575280
- DOI: 10.1016/j.immuni.2015.07.021
MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation
Abstract
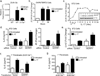
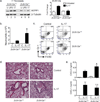
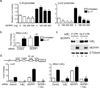
Interleukin-17 (IL-17) induces pathology in autoimmunity and infections; therefore, constraint of this pathway is an essential component of its regulation. We demonstrate that the signaling intermediate MCPIP1 (also termed Regnase-1, encoded by Zc3h12a) is a feedback inhibitor of IL-17 receptor signal transduction. MCPIP1 knockdown enhanced IL-17-mediated signaling, requiring MCPIP1's endoribonuclease but not deubiquitinase domain. MCPIP1 haploinsufficient mice showed enhanced resistance to disseminated Candida albicans infection, which was reversed in an Il17ra(-/-) background. Conversely, IL-17-dependent pathology in Zc3h12a(+/-) mice was exacerbated in both EAE and pulmonary inflammation. MCPIP1 degraded Il6 mRNA directly but only modestly downregulated the IL-6 promoter. However, MCPIP1 strongly inhibited the Lcn2 promoter by regulating the mRNA stability of Nfkbiz, encoding the IκBζ transcription factor. Unexpectedly, MCPIP1 degraded Il17ra and Il17rc mRNA, independently of the 3' UTR. The cumulative impact of MCPIP1 on IL-6, IκBζ, and possibly IL-17R subunits results in a biologically relevant inhibition of IL-17 signaling.
Keywords: IL-17; Regnase-1; autoimmunity; fungal immunity; negative regulation; signal transduction.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Interleukin-17-induced protein lipocalin 2 is dispensable for immunity to oral candidiasis.Infect Immun. 2014 Mar;82(3):1030-5. doi: 10.1128/IAI.01389-13. Epub 2013 Dec 16. Infect Immun. 2014. PMID: 24343647 Free PMC article.
-
MCPIP1/Regnase-1 Restricts IL-17A- and IL-17C-Dependent Skin Inflammation.J Immunol. 2017 Jan 15;198(2):767-775. doi: 10.4049/jimmunol.1601551. Epub 2016 Dec 5. J Immunol. 2017. PMID: 27920272 Free PMC article.
-
Suppression of TCF4 promotes a ZC3H12A-mediated self-sustaining inflammatory feedback cycle involving IL-17RA/IL-17RE epidermal signaling.JCI Insight. 2024 Mar 12;9(8):e172764. doi: 10.1172/jci.insight.172764. JCI Insight. 2024. PMID: 38470486 Free PMC article.
-
IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans.J Immunol. 2015 Aug 1;195(3):780-8. doi: 10.4049/jimmunol.1500909. J Immunol. 2015. PMID: 26188072 Free PMC article. Review.
-
Recent advances in the IL-17 cytokine family.Curr Opin Immunol. 2011 Oct;23(5):613-9. doi: 10.1016/j.coi.2011.07.006. Epub 2011 Aug 16. Curr Opin Immunol. 2011. PMID: 21852080 Free PMC article. Review.
Cited by
-
The IL-17 family in diseases: from bench to bedside.Signal Transduct Target Ther. 2023 Oct 11;8(1):402. doi: 10.1038/s41392-023-01620-3. Signal Transduct Target Ther. 2023. PMID: 37816755 Free PMC article. Review.
-
Arid5a Mediates an IL-17-Dependent Pathway That Drives Autoimmunity but Not Antifungal Host Defense.J Immunol. 2022 Sep 15;209(6):1138-1145. doi: 10.4049/jimmunol.2200132. Epub 2022 Aug 8. J Immunol. 2022. PMID: 35940634 Free PMC article.
-
Dual Role of Act1 in Keratinocyte Differentiation and Host Defense: TRAF3IP2 Silencing Alters Keratinocyte Differentiation and Inhibits IL-17 Responses.J Invest Dermatol. 2017 Jul;137(7):1501-1511. doi: 10.1016/j.jid.2016.12.032. Epub 2017 Mar 6. J Invest Dermatol. 2017. PMID: 28274739 Free PMC article.
-
Monocyte chemotactic protein-induced protein 1 controls allergic airway inflammation by suppressing IL-5-producing TH2 cells through the Notch/Gata3 pathway.J Allergy Clin Immunol. 2018 Aug;142(2):582-594.e10. doi: 10.1016/j.jaci.2017.09.031. Epub 2017 Oct 27. J Allergy Clin Immunol. 2018. PMID: 29111212 Free PMC article.
-
Differential modulation of Ahr and Arid5a: A promising therapeutic strategy for autoimmune encephalomyelitis.Saudi Pharm J. 2020 Dec;28(12):1605-1615. doi: 10.1016/j.jsps.2020.10.007. Epub 2020 Oct 28. Saudi Pharm J. 2020. PMID: 33424253 Free PMC article.
References
-
- Basu S, Quilici C, Zhang HH, Grail D, Dunn AR. Mice lacking both G-CSF and IL-6 are more susceptible to Candida albicans infection: critical role of neutrophils in defense against Candida albicans . Growth Fac. 2008;26:23–34. - PubMed
-
- Camporeale A, Poli V. IL-6, IL-17 and STAT3: A holy trinity in auto-immunity? Front Biosci. 2012;17:2306–2326. - PubMed
-
- Carpenter S, Ricci EP, Mercier BC, Moore MJ, Fitzgerald KA. Post-transcriptional regulation of gene expression in innate immunity. Nat Rev Immunol. 2014;14:361–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE022550/DE/NIDCR NIH HHS/United States
- R01 DE023815/DE/NIDCR NIH HHS/United States
- DE022550/DE/NIDCR NIH HHS/United States
- R56 HL069458/HL/NHLBI NIH HHS/United States
- R01 HL069458/HL/NHLBI NIH HHS/United States
- HL079142/HL/NHLBI NIH HHS/United States
- F32-DE023293/DE/NIDCR NIH HHS/United States
- DE023815/DE/NIDCR NIH HHS/United States
- R37 HL079142/HL/NHLBI NIH HHS/United States
- R37 DE022550/DE/NIDCR NIH HHS/United States
- HL069458/HL/NHLBI NIH HHS/United States
- R01 AI107825/AI/NIAID NIH HHS/United States
- R01 AI110822/AI/NIAID NIH HHS/United States
- F32 DE023293/DE/NIDCR NIH HHS/United States
- R56 AI110822/AI/NIAID NIH HHS/United States
- AI107825/AI/NIAID NIH HHS/United States
- AI110822/AI/NIAID NIH HHS/United States
- R01 HL079142/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

