Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation
- PMID: 26320116
- PMCID: PMC4799677
- DOI: 10.1093/neuonc/nov171
Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation
Abstract
Background: Glioblastoma (GBM) is the most frequent and aggressive primary brain tumor in adults. Recent research on cancer stroma indicates that the brain microenvironment plays a substantial role in tumor malignancy and treatment responses to current antitumor therapy. In this work, we have investigated the effect of alterations in brain tumor extracellular matrix tenascin-C (TNC) on brain tumor growth patterns including proliferation and invasion.
Methods: Since intracranial xenografts from patient-derived GBM neurospheres form highly invasive tumors that recapitulate the invasive features demonstrated in human patients diagnosed with GBM, we studied TNC gain-of-function and loss-of function in these GBM neurospheres in vitro and in vivo.
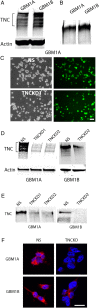
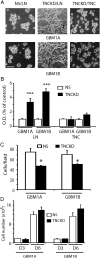
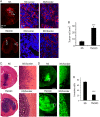
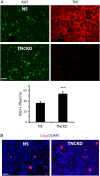
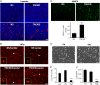
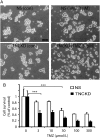
Results: TNC loss-of-function promoted GBM neurosphere cell adhesion and actin cytoskeleton organization. Yet, TNC loss-of-function or exogenous TNC had no effect on GBM neurosphere cell growth in vitro. In animal models, decreased TNC in the tumor microenvironment was accompanied by decreased tumor invasion and increased tumor proliferation, suggesting that TNC regulates the "go-or-grow" phenotypic switch of glioma in vivo. We demonstrated that decreased TNC in the tumor microenvironment modulated behaviors of stromal cells including endothelial cells and microglia, resulting in enlarged tumor blood vessels and activated microglia in tumors. We further demonstrated that tumor cells with decreased TNC expression are sensitive to anti-proliferative treatment in vitro.
Conclusion: Our findings suggest that detailed understanding of how TNC in the tumor microenvironment influences tumor behavior and the interactions between tumor cells and surrounding nontumor cells will benefit novel combinatory antitumor strategies to treat malignant brain tumors.
Keywords: TNC; glioblastoma; patient-derived GBM neurospheres; tumor microenvironment.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
ADAM-9 is a novel mediator of tenascin-C-stimulated invasiveness of brain tumor-initiating cells.Neuro Oncol. 2015 Aug;17(8):1095-105. doi: 10.1093/neuonc/nou362. Epub 2015 Feb 1. Neuro Oncol. 2015. PMID: 25646025 Free PMC article.
-
Tenascin-C: a novel candidate marker for cancer stem cells in glioblastoma identified by tissue microarrays.J Proteome Res. 2015 Feb 6;14(2):814-22. doi: 10.1021/pr5008653. Epub 2014 Dec 12. J Proteome Res. 2015. PMID: 25469866 Free PMC article.
-
EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase.Oncogene. 2012 Dec 13;31(50):5132-43. doi: 10.1038/onc.2012.16. Epub 2012 Feb 6. Oncogene. 2012. PMID: 22310282 Free PMC article.
-
Biologically Active TNIIIA2 Region in Tenascin-C Molecule: A Major Contributor to Elicit Aggressive Malignant Phenotypes From Tumors/Tumor Stroma.Front Immunol. 2020 Dec 9;11:610096. doi: 10.3389/fimmu.2020.610096. eCollection 2020. Front Immunol. 2020. PMID: 33362799 Free PMC article. Review.
-
Tenascin-C Function in Glioma: Immunomodulation and Beyond.Adv Exp Med Biol. 2020;1272:149-172. doi: 10.1007/978-3-030-48457-6_9. Adv Exp Med Biol. 2020. PMID: 32845507 Review.
Cited by
-
Cellular Plasticity and Tumor Microenvironment in Gliomas: The Struggle to Hit a Moving Target.Cancers (Basel). 2020 Jun 18;12(6):1622. doi: 10.3390/cancers12061622. Cancers (Basel). 2020. PMID: 32570988 Free PMC article. Review.
-
GBM-Targeted oHSV Armed with Matrix Metalloproteinase 9 Enhances Anti-tumor Activity and Animal Survival.Mol Ther Oncolytics. 2019 Oct 24;15:214-222. doi: 10.1016/j.omto.2019.10.005. eCollection 2019 Dec 20. Mol Ther Oncolytics. 2019. PMID: 31890868 Free PMC article.
-
Gene signatures of quiescent glioblastoma cells reveal mesenchymal shift and interactions with niche microenvironment.EBioMedicine. 2019 Apr;42:252-269. doi: 10.1016/j.ebiom.2019.03.064. Epub 2019 Apr 3. EBioMedicine. 2019. PMID: 30952620 Free PMC article.
-
A role for GLUT3 in glioblastoma cell invasion that is not recapitulated by GLUT1.Cell Adh Migr. 2021 Dec;15(1):101-115. doi: 10.1080/19336918.2021.1903684. Cell Adh Migr. 2021. PMID: 33843470 Free PMC article.
-
From Bowen disease to cutaneous squamous cell carcinoma: eight markers were verified from transcriptomic and proteomic analyses.J Transl Med. 2022 Sep 9;20(1):416. doi: 10.1186/s12967-022-03622-1. J Transl Med. 2022. PMID: 36085041 Free PMC article.
References
-
- Charles NA, Holland EC, Gilbertson R, et al. The brain tumor microenvironment. Glia. 2011;59(8):1169–1180. - PubMed
-
- Quirico-Santos T, Fonseca CO, Lagrota-Candido J. Brain sweet brain: importance of sugars for the cerebral microenvironment and tumor development. Arq Neuropsiquiatr. 2010;68(5):799–803. - PubMed
-
- Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218(2):235–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

