Susceptibility of bone marrow-derived macrophages to influenza virus infection is dependent on macrophage phenotype
- PMID: 26297234
- PMCID: PMC4635478
- DOI: 10.1099/jgv.0.000240
Susceptibility of bone marrow-derived macrophages to influenza virus infection is dependent on macrophage phenotype
Abstract
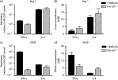
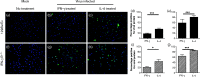
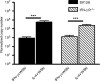
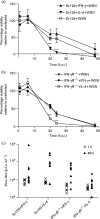
The role of the macrophage in influenza virus infection is complex. Macrophages are critical for resolution of influenza virus infections but implicated in morbidity and mortality in severe infections. They can be infected with influenza virus and consequently macrophage infection is likely to have an impact on the host immune response. Macrophages display a range of functional phenotypes, from the prototypical pro-inflammatory classically activated cell to alternatively activated anti-inflammatory macrophages involved in immune regulation and wound healing. We were interested in how macrophages of different phenotype respond to influenza virus infection and therefore studied the infection of bone marrow-derived macrophages (BMDMs) of classical and alternative phenotype in vitro. Our results show that alternatively activated macrophages are more readily infected and killed by the virus than classically activated. Classically activated BMDMs express the pro-inflammatory markers inducible nitric oxide synthase (iNOS) and TNF-α, and TNF-α expression was further upregulated following infection. Alternatively activated macrophages express Arginase-1 and CD206; however, following infection, expression of these markers was downregulated whilst expression of iNOS and TNF-α was upregulated. Thus, infection can override the anti-inflammatory state of alternatively activated macrophages. Importantly, however, this results in lower levels of pro-inflammatory markers than those produced by classically activated cells. Our results showed that macrophage phenotype affects the inflammatory macrophage response following infection, and indicated that modulating the macrophage phenotype may provide a route to develop novel strategies to prevent and treat influenza virus infection.
Figures


Similar articles
-
CLEC5A-Mediated Enhancement of the Inflammatory Response in Myeloid Cells Contributes to Influenza Virus Pathogenicity In Vivo.J Virol. 2016 Dec 16;91(1):e01813-16. doi: 10.1128/JVI.01813-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795434 Free PMC article.
-
Spleen-derived macrophages are readily polarized into classically activated (M1) or alternatively activated (M2) states.Immunobiology. 2014 Oct;219(10):737-45. doi: 10.1016/j.imbio.2014.05.005. Epub 2014 Jun 6. Immunobiology. 2014. PMID: 24954891
-
Fibronectin aggregates promote features of a classically and alternatively activated phenotype in macrophages.J Neuroinflammation. 2018 Aug 2;15(1):218. doi: 10.1186/s12974-018-1238-x. J Neuroinflammation. 2018. PMID: 30071854 Free PMC article.
-
Influenza virus replication in macrophages: balancing protection and pathogenesis.J Gen Virol. 2017 Oct;98(10):2401-2412. doi: 10.1099/jgv.0.000922. Epub 2017 Sep 8. J Gen Virol. 2017. PMID: 28884667 Free PMC article. Review.
-
Macrophage phenotype in response to ECM bioscaffolds.Semin Immunol. 2017 Feb;29:2-13. doi: 10.1016/j.smim.2017.04.004. Epub 2017 Jul 21. Semin Immunol. 2017. PMID: 28736160 Free PMC article. Review.
Cited by
-
Rubella Virus Strain-Associated Differences in the Induction of Oxidative Stress Are Independent of Their Interferon Activation.Viruses. 2018 Oct 3;10(10):540. doi: 10.3390/v10100540. Viruses. 2018. PMID: 30282907 Free PMC article.
-
Lack of IFNγ signaling attenuates spread of influenza A virus in vivo and leads to reduced pathogenesis.Virology. 2019 Jan 2;526:155-164. doi: 10.1016/j.virol.2018.10.017. Epub 2018 Oct 31. Virology. 2019. PMID: 30390564 Free PMC article.
-
Influenza, SARS-CoV-2, and Their Impact on Chronic Lung Diseases and Fibrosis: Exploring Therapeutic Options.Am J Pathol. 2024 Oct;194(10):1807-1822. doi: 10.1016/j.ajpath.2024.06.004. Epub 2024 Jul 18. Am J Pathol. 2024. PMID: 39032604 Review.
-
Distinct Chronic Post-Viral Lung Diseases upon Infection with Influenza or Parainfluenza Viruses Differentially Impact Superinfection Outcome.Am J Pathol. 2020 Mar;190(3):543-553. doi: 10.1016/j.ajpath.2019.11.003. Epub 2019 Dec 19. Am J Pathol. 2020. PMID: 31866346 Free PMC article.
-
The induction and consequences of Influenza A virus-induced cell death.Cell Death Dis. 2018 Sep 25;9(10):1002. doi: 10.1038/s41419-018-1035-6. Cell Death Dis. 2018. PMID: 30254192 Free PMC article. Review.
References
-
- Cheung C.Y., Poon L.L.M., Lau A.S., Luk W., Lau Y.L., Shortridge K.F., Gordon S., Guan Y., Peiris J.S.M. (2002). Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360 1831–1837 10.1016/S0140-6736(02)11772-7 . - DOI - PubMed
-
- Gao J.J., Filla M.B., Fultz M.J., Vogel S.N., Russell S.W., Murphy W.J. (1998). Autocrine/paracrine IFN-alphabeta mediates the lipopolysaccharide-induced activation of transcription factor Stat1alpha in mouse macrophages: pivotal role of Stat1alpha in induction of the inducible nitric oxide synthase gene J Immunol 161 4803–4810 . - PubMed
-
- Geller D.A., de Vera M.E., Russell D.A., Shapiro R.A., Nussler A.K., Simmons R.L., Billiar T.R. (1995). A central role for IL-1 beta in the in vitro and in vivo regulation of hepatic inducible nitric oxide synthase. IL-1 beta induces hepatic nitric oxide synthesis J Immunol 155 4890–4898 . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

