Structural characterization of muropeptides from Chlamydia trachomatis peptidoglycan by mass spectrometry resolves "chlamydial anomaly"
- PMID: 26290580
- PMCID: PMC4577195
- DOI: 10.1073/pnas.1514026112
Structural characterization of muropeptides from Chlamydia trachomatis peptidoglycan by mass spectrometry resolves "chlamydial anomaly"
Abstract
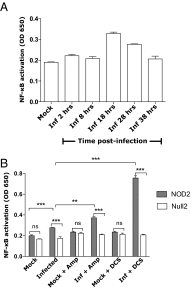
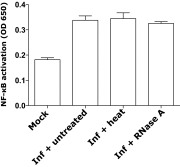
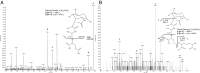
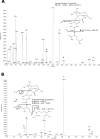
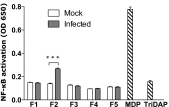
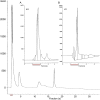
The "chlamydial anomaly," first coined by James Moulder, describes the inability of researchers to detect or purify peptidoglycan (PG) from pathogenic Chlamydiae despite genetic and biochemical evidence and antibiotic susceptibility data that suggest its existence. We recently detected PG in Chlamydia trachomatis by a new metabolic cell wall labeling method, however efforts to purify PG from pathogenic Chlamydiae have remained unsuccessful. Pathogenic chlamydial species are known to activate nucleotide-binding oligomerization domain-containing protein 2 (NOD2) innate immune receptors by as yet uncharacterized ligands, which are presumed to be PG fragments (muramyl di- and tripeptides). We used the NOD2-dependent activation of NF-κB by C. trachomatis-infected cell lysates as a biomarker for the presence of PG fragments within specific lysate fractions. We designed a new method of muropeptide isolation consisting of a double filtration step coupled with reverse-phase HPLC fractionation of Chlamydia-infected HeLa cell lysates. Fractions that displayed NOD2 activity were analyzed by electrospray ionization mass spectrometry, confirming the presence of muramyl di- and tripeptides in Chlamydia-infected cell lysate fractions. Moreover, the mass spectrometry data of large muropeptide fragments provided evidence that transpeptidation and transglycosylation reactions occur in pathogenic Chlamydiae. These results reveal the composition of chlamydial PG and disprove the "glycanless peptidoglycan" hypothesis.
Keywords: NOD2 receptor; chlamydia; mass spectrometry; muropeptide; peptidoglycan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis.Nature. 2014 Feb 27;506(7489):507-10. doi: 10.1038/nature12892. Epub 2013 Dec 11. Nature. 2014. PMID: 24336210 Free PMC article.
-
In vitro and in vivo functional activity of Chlamydia MurA, a UDP-N-acetylglucosamine enolpyruvyl transferase involved in peptidoglycan synthesis and fosfomycin resistance.J Bacteriol. 2003 Feb;185(4):1218-28. doi: 10.1128/JB.185.4.1218-1228.2003. J Bacteriol. 2003. PMID: 12562791 Free PMC article.
-
The chlamydial anomaly clarified?Chembiochem. 2014 Jul 7;15(10):1391-2. doi: 10.1002/cbic.201402143. Epub 2014 Jun 2. Chembiochem. 2014. PMID: 24891214
-
Why is Chlamydia sensitive to penicillin in the absence of peptidoglycan?Infect Agents Dis. 1993 Apr;2(2):87-99. Infect Agents Dis. 1993. PMID: 8162358 Review.
-
The role of NOD1 and NOD2 in host defense against chlamydial infection.FEMS Microbiol Lett. 2016 Sep;363(17):fnw170. doi: 10.1093/femsle/fnw170. Epub 2016 Jul 14. FEMS Microbiol Lett. 2016. PMID: 27421958 Review.
Cited by
-
Chlamydia cell biology and pathogenesis.Nat Rev Microbiol. 2016 Jun;14(6):385-400. doi: 10.1038/nrmicro.2016.30. Epub 2016 Apr 25. Nat Rev Microbiol. 2016. PMID: 27108705 Free PMC article. Review.
-
Chlamydial Antibiotic Resistance and Treatment Failure in Veterinary and Human Medicine.Curr Clin Microbiol Rep. 2016;3:10-18. doi: 10.1007/s40588-016-0028-4. Epub 2016 Feb 3. Curr Clin Microbiol Rep. 2016. PMID: 27218014 Free PMC article. Review.
-
Bacterial peptidoglycan muropeptides benefit mitochondrial homeostasis and animal physiology by acting as ATP synthase agonists.Dev Cell. 2022 Feb 7;57(3):361-372.e5. doi: 10.1016/j.devcel.2021.12.016. Epub 2022 Jan 18. Dev Cell. 2022. PMID: 35045336 Free PMC article.
-
Bacterial Cell Division: Nonmodels Poised to Take the Spotlight.Annu Rev Microbiol. 2017 Sep 8;71:393-411. doi: 10.1146/annurev-micro-102215-095657. Epub 2017 Jul 11. Annu Rev Microbiol. 2017. PMID: 28697666 Free PMC article. Review.
-
Bacillus anthracis Peptidoglycan Integrity Is Disrupted by the Chemokine CXCL10 through the FtsE/X Complex.Front Microbiol. 2017 Apr 27;8:740. doi: 10.3389/fmicb.2017.00740. eCollection 2017. Front Microbiol. 2017. PMID: 28496437 Free PMC article.
References
-
- Stephens RS, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282(5389):754–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

