Dexamethasone-Mediated Activation of Fibronectin Matrix Assembly Reduces Dispersal of Primary Human Glioblastoma Cells
- PMID: 26284619
- PMCID: PMC4540426
- DOI: 10.1371/journal.pone.0135951
Dexamethasone-Mediated Activation of Fibronectin Matrix Assembly Reduces Dispersal of Primary Human Glioblastoma Cells
Abstract
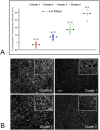
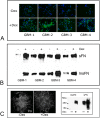
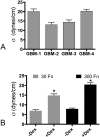
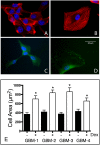
Despite resection and adjuvant therapy, the 5-year survival for patients with Glioblastoma multiforme (GBM) is less than 10%. This poor outcome is largely attributed to rapid tumor growth and early dispersal of cells, factors that contribute to a high recurrence rate and poor prognosis. An understanding of the cellular and molecular machinery that drive growth and dispersal is essential if we are to impact long-term survival. Our previous studies utilizing a series of immortalized GBM cell lines established a functional causation between activation of fibronectin matrix assembly (FNMA), increased tumor cohesion, and decreased dispersal. Activation of FNMA was accomplished by treatment with Dexamethasone (Dex), a drug routinely used to treat brain tumor related edema. Here, we utilize a broad range of qualitative and quantitative assays and the use of a human GBM tissue microarray and freshly-isolated primary human GBM cells grown both as conventional 2D cultures and as 3D spheroids to explore the role of Dex and FNMA in modulating various parameters that can significantly influence tumor cell dispersal. We show that the expression and processing of fibronectin in a human GBM tissue-microarray is variable, with 90% of tumors displaying some abnormality or lack in capacity to secrete fibronectin or assemble it into a matrix. We also show that low-passage primary GBM cells vary in their capacity for FNMA and that Dex treatment reactivates this process. Activation of FNMA effectively "glues" cells together and prevents cells from detaching from the primary mass. Dex treatment also significantly increases the strength of cell-ECM adhesion and decreases motility. The combination of increased cohesion and decreased motility discourages in vitro and ex vivo dispersal. By increasing cell-cell cohesion, Dex also decreases growth rate of 3D spheroids. These effects could all be reversed by an inhibitor of FNMA and by the glucocorticoid receptor antagonist, RU-486. Our results describe a new role for Dex as a suppressor of GBM dispersal and growth.
Conflict of interest statement
Figures
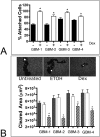
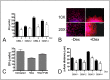
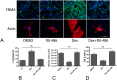
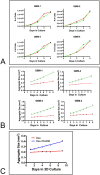
Similar articles
-
Dexamethasone-Mediated Upregulation of Calreticulin Inhibits Primary Human Glioblastoma Dispersal Ex Vivo.Int J Mol Sci. 2018 Feb 14;19(2):572. doi: 10.3390/ijms19020572. Int J Mol Sci. 2018. PMID: 29443896 Free PMC article.
-
Fibronectin matrix assembly suppresses dispersal of glioblastoma cells.PLoS One. 2011;6(9):e24810. doi: 10.1371/journal.pone.0024810. Epub 2011 Sep 30. PLoS One. 2011. PMID: 21980357 Free PMC article.
-
Teaching an Old Drug New Tricks: Dexamethasone as an In Vivo Inhibitor of Glioblastoma Dispersal.Cureus. 2020 Apr 20;12(4):e7749. doi: 10.7759/cureus.7749. Cureus. 2020. PMID: 32455066 Free PMC article.
-
Biologically Active TNIIIA2 Region in Tenascin-C Molecule: A Major Contributor to Elicit Aggressive Malignant Phenotypes From Tumors/Tumor Stroma.Front Immunol. 2020 Dec 9;11:610096. doi: 10.3389/fimmu.2020.610096. eCollection 2020. Front Immunol. 2020. PMID: 33362799 Free PMC article. Review.
-
Advantages and drawbacks of dexamethasone in glioblastoma multiforme.Crit Rev Oncol Hematol. 2022 Apr;172:103625. doi: 10.1016/j.critrevonc.2022.103625. Epub 2022 Feb 11. Crit Rev Oncol Hematol. 2022. PMID: 35158070 Review.
Cited by
-
Systemic high-dose dexamethasone treatment may modulate the efficacy of intratumoral viral oncolytic immunotherapy in glioblastoma models.J Immunother Cancer. 2022 Jan;10(1):e003368. doi: 10.1136/jitc-2021-003368. J Immunother Cancer. 2022. PMID: 35017150 Free PMC article.
-
Role of TGF-β1/SMADs signalling pathway in resveratrol-induced reduction of extracellular matrix deposition by dexamethasone-treated human trabecular meshwork cells.Korean J Physiol Pharmacol. 2024 Jul 1;28(4):345-359. doi: 10.4196/kjpp.2024.28.4.345. Korean J Physiol Pharmacol. 2024. PMID: 38926842 Free PMC article.
-
Glucose Influences the Response of Glioblastoma Cells to Temozolomide and Dexamethasone.Cancer Control. 2022 Jan-Dec;29:10732748221075468. doi: 10.1177/10732748221075468. Cancer Control. 2022. PMID: 35225010 Free PMC article.
-
Conventional Anti-glioblastoma Chemotherapy Affects Proteoglycan Composition of Brain Extracellular Matrix in Rat Experimental Model in vivo.Front Pharmacol. 2018 Oct 2;9:1104. doi: 10.3389/fphar.2018.01104. eCollection 2018. Front Pharmacol. 2018. PMID: 30333749 Free PMC article.
-
Dexamethasone Inhibits Spheroid Formation of Thyroid Cancer Cells Exposed to Simulated Microgravity.Cells. 2020 Feb 5;9(2):367. doi: 10.3390/cells9020367. Cells. 2020. PMID: 32033410 Free PMC article.
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10(5):459–66. 10.1016/S1470-2045(09)70025-7 . - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

