The role of Fc-FcγR interactions in IgG-mediated microbial neutralization
- PMID: 26282878
- PMCID: PMC4548051
- DOI: 10.1084/jem.20151267
The role of Fc-FcγR interactions in IgG-mediated microbial neutralization
Abstract
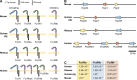
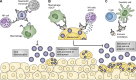
Antibodies are bifunctional molecules, containing a variable Fab domain that mediates binding specificity and a constant Fc domain that bridges antibody-coated targets with FcγR-expressing cells that mediate effector functions. Although traditional mechanisms of antibody-mediated neutralization of microbes have been largely thought to result from Fab-antigen interactions, recent studies suggest that recruitment of FcγR-expressing effector cells by antibodies is a major in vivo mechanism of antibody-mediated protection from infection. In this article, we review FcγR biology, compare mammalian FcγR families, and summarize recent evidence demonstrating the crucial role that Fc-FcγR interactions play during in vivo protection from infection.
© 2015 Bournazos et al.
Figures
Similar articles
-
Divergent Requirement of Fc-Fcγ Receptor Interactions for In Vivo Protection against Influenza Viruses by Two Pan-H5 Hemagglutinin Antibodies.J Virol. 2017 May 12;91(11):e02065-16. doi: 10.1128/JVI.02065-16. Print 2017 Jun 1. J Virol. 2017. PMID: 28331095 Free PMC article.
-
Anti-retroviral antibody FcγR-mediated effector functions.Immunol Rev. 2017 Jan;275(1):285-295. doi: 10.1111/imr.12482. Immunol Rev. 2017. PMID: 28133801 Free PMC article. Review.
-
Differential requirements for FcγR engagement by protective antibodies against Ebola virus.Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):20054-20062. doi: 10.1073/pnas.1911842116. Epub 2019 Sep 4. Proc Natl Acad Sci U S A. 2019. PMID: 31484758 Free PMC article.
-
Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms.J Reprod Immunol. 2008 Jun;78(1):58-67. doi: 10.1016/j.jri.2007.08.004. Epub 2007 Oct 24. J Reprod Immunol. 2008. PMID: 17950908 Free PMC article.
-
Fc receptor-mediated antiviral antibodies.Curr Opin HIV AIDS. 2009 Sep;4(5):388-93. doi: 10.1097/COH.0b013e32832f0a89. Curr Opin HIV AIDS. 2009. PMID: 20048702 Free PMC article. Review.
Cited by
-
Prospects and Challenges in the Development of Universal Influenza Vaccines.Vaccines (Basel). 2020 Jul 6;8(3):361. doi: 10.3390/vaccines8030361. Vaccines (Basel). 2020. PMID: 32640619 Free PMC article. Review.
-
Recent findings on the Coronavirus disease 2019 (COVID-19); immunopathogenesis and immunotherapeutics.Int Immunopharmacol. 2020 Dec;89(Pt B):107082. doi: 10.1016/j.intimp.2020.107082. Epub 2020 Oct 10. Int Immunopharmacol. 2020. PMID: 33068865 Free PMC article. Review.
-
Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs.Front Immunol. 2020 Jun 9;11:1100. doi: 10.3389/fimmu.2020.01100. eCollection 2020. Front Immunol. 2020. PMID: 32582186 Free PMC article. Review.
-
Fc Gamma Receptor Polymorphisms Modulated the Vaccine Effect on HIV-1 Risk in the HVTN 505 HIV Vaccine Trial.J Virol. 2019 Oct 15;93(21):e02041-18. doi: 10.1128/JVI.02041-18. Print 2019 Nov 1. J Virol. 2019. PMID: 31434737 Free PMC article.
-
Antibodies to a Conserved Influenza Head Interface Epitope Protect by an IgG Subtype-Dependent Mechanism.Cell. 2019 May 16;177(5):1124-1135.e16. doi: 10.1016/j.cell.2019.03.048. Cell. 2019. PMID: 31100267 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

