Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity
- PMID: 26282538
- PMCID: PMC4539932
- DOI: 10.1186/s13287-015-0137-7
Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity
Abstract
Introduction: Previously, we established a simple method for deriving mesenchymal stem cells (MSCs) from human induced pluripotent stem cells (iPSC-MSCs). These iPSC-MSCs were capable of forming osteogenic structures in scaffolds and nanofibers. The objective of this study is to systematically characterize the mesenchymal characteristics of the iPSC-MSCs by comparing them to bone marrow-derived MSCs (BM-MSCs).
Methods: Two iPSC-MSC lines (named as mRNA-iPSC-MSC-YL001 and lenti-iPSC-MSC-A001) and one BM-MSC line were used for the study. Cell proliferation, presence of mesenchymal surface markers, tri-lineage differentiation capability (osteogenesis, chondrogenesis, adipogenesis), and expression of "stemness" genes were analyzed in these MSC lines.
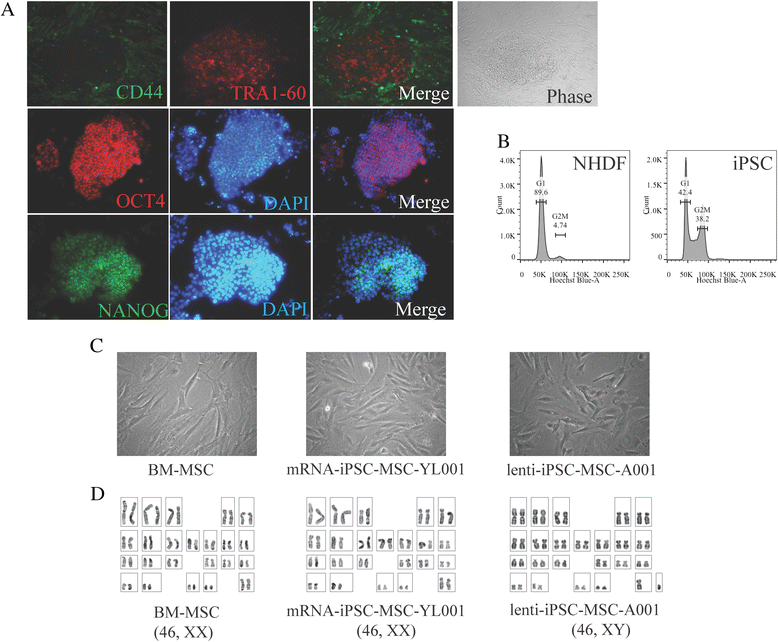
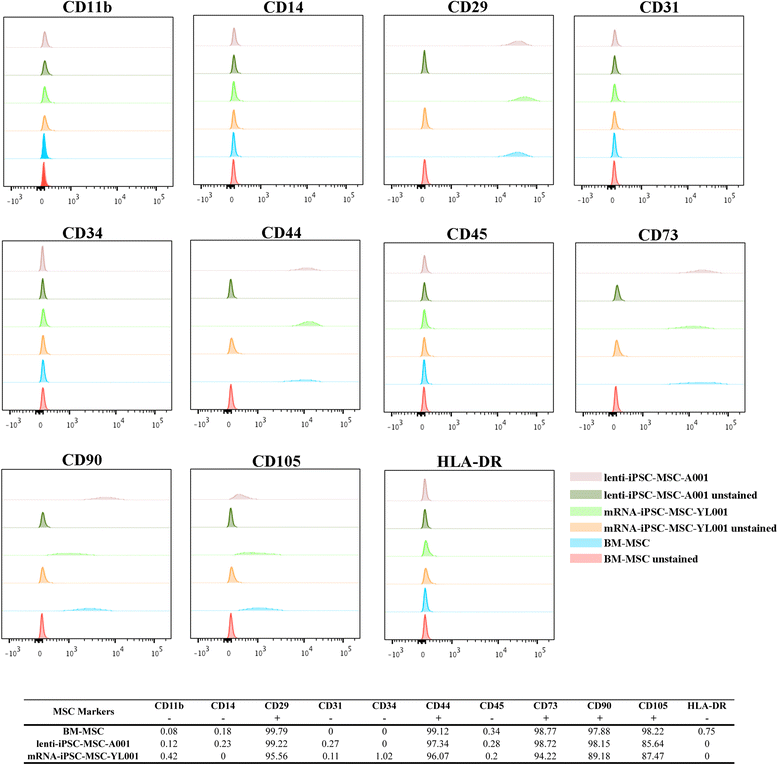
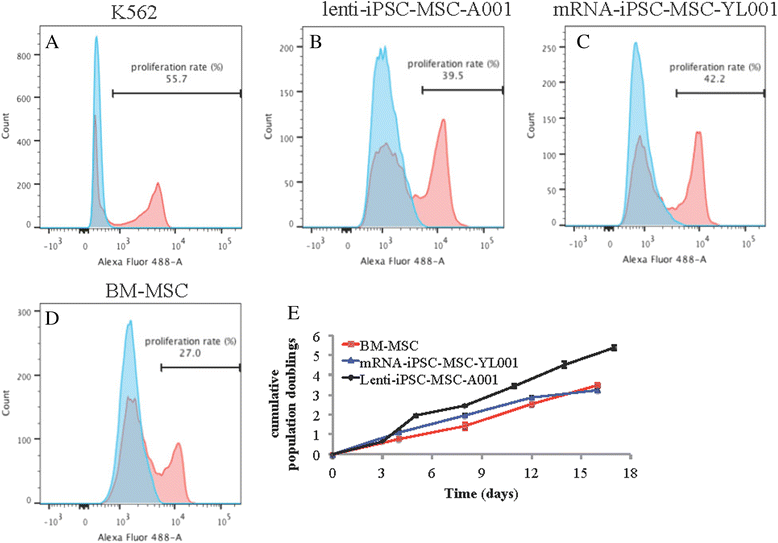
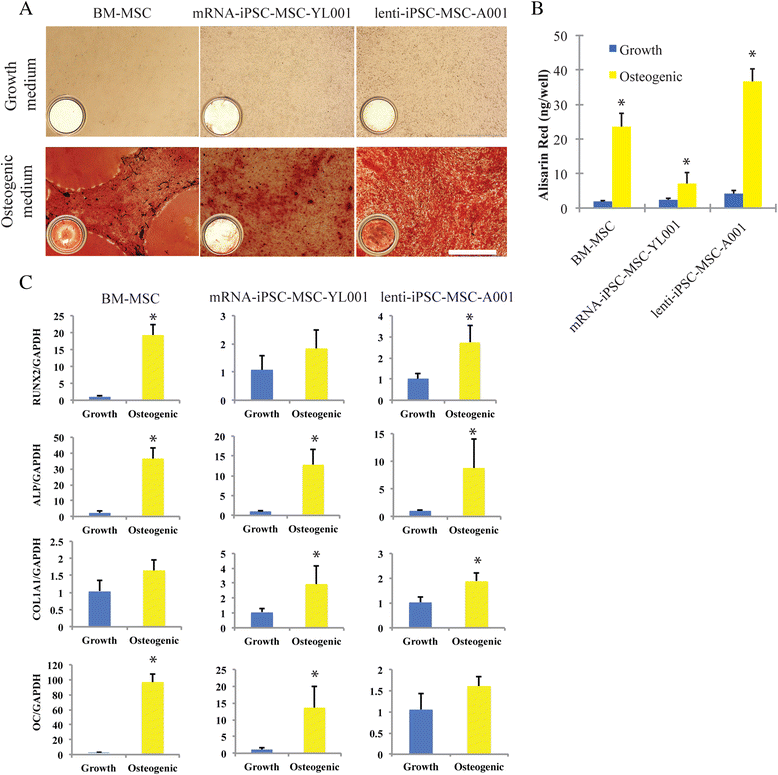
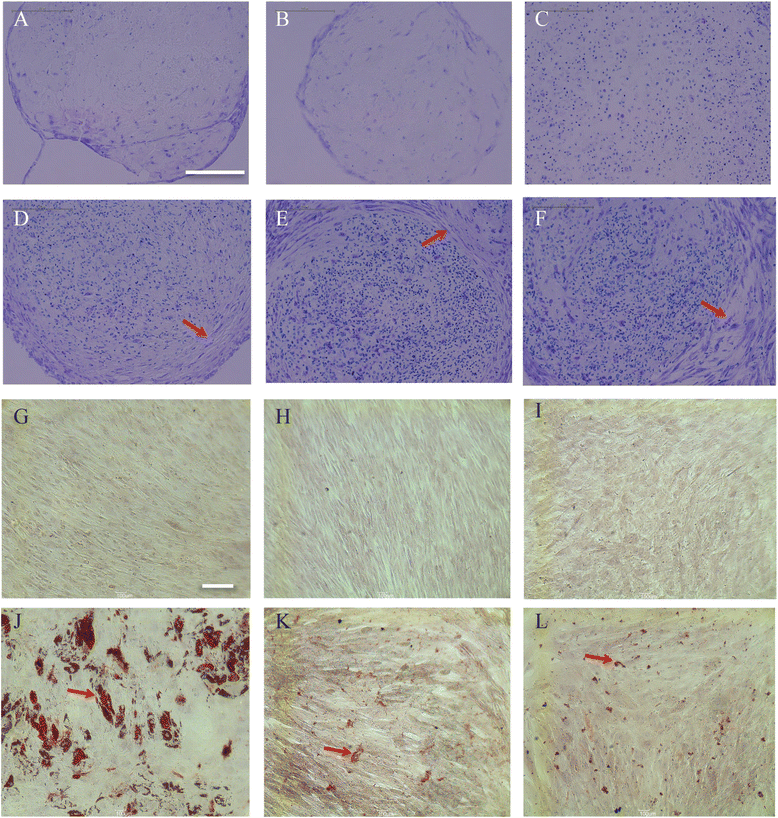
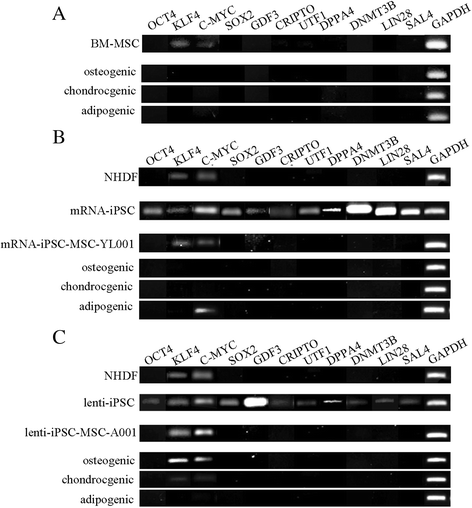
Results: The iPSC-MSCs were similar to BM-MSCs in terms of cell morphology (fibroblast-like) and surface antigen profile: CD29+, CD44+, CD73+, CD90+, CD105+, CD11b-, CD14-, CD31-, CD34-, CD45- and HLA-DR-. A faster proliferative capability was seen in both iPSC-MSCs lines compared to the BM-MSCs. The iPSC-MSCs showed adequate capacity of osteogenesis and chondrogenesis compared to the BM-MSCs, while less adipogenic potential was found in the iPSC-MSCs. The iPSC-MSCs and the tri-lineage differentiated cells (osteoblasts, chondrocytes, adipocytes) all lack expression of "stemness" genes: OCT4, SOX2, GDF3, CRIPTO, UTF1, DPPA4, DNMT3B, LIN28a, and SAL4.
Conclusions: The MSCs derived from human iPSCs with our method have advanced proliferation capability and adequate osteogenic and chondrogenic properties compared to BM-MSCs. However, the iPSC-MSCs were less efficient in their adipogenicity, suggesting that further modifications should be applied to our method to derive iPSC-MSCs more closely resembling the naïve BM-MSCs if necessary.
Figures
Similar articles
-
Mesenchymal Stem Cells Obtained from Synovial Fluid Mesenchymal Stem Cell-Derived Induced Pluripotent Stem Cells on a Matrigel Coating Exhibited Enhanced Proliferation and Differentiation Potential.PLoS One. 2015 Dec 9;10(12):e0144226. doi: 10.1371/journal.pone.0144226. eCollection 2015. PLoS One. 2015. PMID: 26649753 Free PMC article.
-
Prospectively Isolated Human Bone Marrow Cell-Derived MSCs Support Primitive Human CD34-Negative Hematopoietic Stem Cells.Stem Cells. 2015 May;33(5):1554-65. doi: 10.1002/stem.1941. Stem Cells. 2015. PMID: 25537923
-
Generation, Characterization, and Multilineage Potency of Mesenchymal-Like Progenitors Derived from Equine Induced Pluripotent Stem Cells.Stem Cells Dev. 2016 Jan 1;25(1):80-9. doi: 10.1089/scd.2014.0409. Epub 2015 Nov 5. Stem Cells Dev. 2016. PMID: 26414480
-
Dynamic changes of epigenetic signatures during chondrogenic and adipogenic differentiation of mesenchymal stem cells.Biomed Pharmacother. 2017 May;89:719-731. doi: 10.1016/j.biopha.2017.02.093. Epub 2017 Mar 6. Biomed Pharmacother. 2017. PMID: 28273634 Review.
-
The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells.Protein Cell. 2017 Jun;8(6):439-445. doi: 10.1007/s13238-017-0385-7. Epub 2017 Mar 7. Protein Cell. 2017. PMID: 28271444 Free PMC article. Review.
Cited by
-
Stem cell-based therapies for tumors in the brain: are we there yet?Neuro Oncol. 2016 Aug;18(8):1066-78. doi: 10.1093/neuonc/now096. Epub 2016 Jun 9. Neuro Oncol. 2016. PMID: 27282399 Free PMC article. Review.
-
The Possible Roles of Biological Bone Constructed with Peripheral Blood Derived EPCs and BMSCs in Osteogenesis and Angiogenesis.Biomed Res Int. 2016;2016:8168943. doi: 10.1155/2016/8168943. Epub 2016 Apr 18. Biomed Res Int. 2016. PMID: 27195296 Free PMC article.
-
Differentiation of equine induced pluripotent stem cells into mesenchymal lineage for therapeutic use.Cell Cycle. 2019 Nov;18(21):2954-2971. doi: 10.1080/15384101.2019.1664224. Epub 2019 Sep 11. Cell Cycle. 2019. PMID: 31505996 Free PMC article.
-
INDUCED PLURIPOTENT STEM CELL-DERIVED MESENCHYMAL STEM CELLS-DERIVED EXTRACELLULAR VESICLES ATTENUATE LPS-INDUCED LUNG INJURY AND ENDOTOXEMIA IN MICE.Shock. 2024 Aug 1;62(2):294-303. doi: 10.1097/SHK.0000000000002381. Epub 2024 May 23. Shock. 2024. PMID: 38813932
-
Reprogramming of blood cells into induced pluripotent stem cells as a new cell source for cartilage repair.Stem Cell Res Ther. 2016 Feb 17;7:31. doi: 10.1186/s13287-016-0290-7. Stem Cell Res Ther. 2016. PMID: 26883322 Free PMC article.
References
-
- Kang R, Luo Y, Zou L, Xie L, Lysdahl H, Jiang X, et al. Osteogenesis of human induced pluripotent stem cells derived mesenchymal stem cells on hydroxyapatite contained nanofibers. RSC Adv. 2014;4:5734–9. doi: 10.1039/c3ra44181d. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

