Alternative Wnt Signaling Activates YAP/TAZ
- PMID: 26276632
- PMCID: PMC4538707
- DOI: 10.1016/j.cell.2015.07.013
Alternative Wnt Signaling Activates YAP/TAZ
Abstract
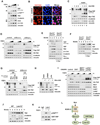
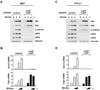
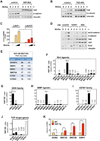
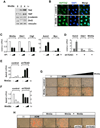
The transcriptional co-activators YAP and TAZ are key regulators of organ size and tissue homeostasis, and their dysregulation contributes to human cancer. Here, we discover YAP/TAZ as bona fide downstream effectors of the alternative Wnt signaling pathway. Wnt5a/b and Wnt3a induce YAP/TAZ activation independent of canonical Wnt/β-catenin signaling. Mechanistically, we delineate the "alternative Wnt-YAP/TAZ signaling axis" that consists of Wnt-FZD/ROR-Gα12/13-Rho GTPases-Lats1/2 to promote YAP/TAZ activation and TEAD-mediated transcription. YAP/TAZ mediate the biological functions of alternative Wnt signaling, including gene expression, osteogenic differentiation, cell migration, and antagonism of Wnt/β-catenin signaling. Together, our work establishes YAP/TAZ as critical mediators of alternative Wnt signaling.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response.Cell. 2014 Jul 3;158(1):157-70. doi: 10.1016/j.cell.2014.06.013. Epub 2014 Jun 26. Cell. 2014. PMID: 24976009
-
The YAP/TAZ transcriptional co-activators have opposing effects at different stages of osteoblast differentiation.Bone. 2018 Jul;112:1-9. doi: 10.1016/j.bone.2018.04.001. Epub 2018 Apr 4. Bone. 2018. PMID: 29626544 Free PMC article.
-
Wnt/β-catenin signaling via Axin2 is required for myogenesis and, together with YAP/Taz and Tead1, active in IIa/IIx muscle fibers.Development. 2016 Sep 1;143(17):3128-42. doi: 10.1242/dev.139907. Development. 2016. PMID: 27578179
-
Multifaceted regulation and functions of YAP/TAZ in tumors (Review).Oncol Rep. 2018 Jul;40(1):16-28. doi: 10.3892/or.2018.6423. Epub 2018 May 8. Oncol Rep. 2018. PMID: 29749524 Free PMC article. Review.
-
YAP/TAZ for cancer therapy: opportunities and challenges (review).Int J Oncol. 2015 Apr;46(4):1444-52. doi: 10.3892/ijo.2015.2877. Epub 2015 Feb 5. Int J Oncol. 2015. PMID: 25652178 Review.
Cited by
-
β-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis.Genes Dev. 2015 Jul 15;29(14):1493-506. doi: 10.1101/gad.264515.115. Epub 2015 Jul 20. Genes Dev. 2015. PMID: 26193883 Free PMC article.
-
LncRNA-CCAT5-mediated crosstalk between Wnt/β-Catenin and STAT3 signaling suggests novel therapeutic approaches for metastatic gastric cancer with high Wnt activity.Cancer Commun (Lond). 2024 Jan;44(1):76-100. doi: 10.1002/cac2.12507. Epub 2023 Nov 27. Cancer Commun (Lond). 2024. PMID: 38010289 Free PMC article.
-
Loss of RET Promotes Mesenchymal Identity in Neuroblastoma Cells.Cancers (Basel). 2021 Apr 15;13(8):1909. doi: 10.3390/cancers13081909. Cancers (Basel). 2021. PMID: 33921066 Free PMC article.
-
Decreased extracellular pH inhibits osteogenesis through proton-sensing GPR4-mediated suppression of yes-associated protein.Sci Rep. 2016 Jun 3;6:26835. doi: 10.1038/srep26835. Sci Rep. 2016. PMID: 27256071 Free PMC article.
-
Frizzled Receptors in Tumors, Focusing on Signaling, Roles, Modulation Mechanisms, and Targeted Therapies.Oncol Res. 2021 Mar 16;28(6):661-674. doi: 10.3727/096504020X16014648664459. Epub 2020 Sep 30. Oncol Res. 2021. PMID: 32998794 Free PMC article. Review.
References
-
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. - PubMed
-
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. - PubMed
-
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ Incorporation in the beta-Catenin Destruction Complex Orchestrates the Wnt Response. Cell. 2014;158:157–170. - PubMed
-
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

