T cell metabolism drives immunity
- PMID: 26261266
- PMCID: PMC4548052
- DOI: 10.1084/jem.20151159
T cell metabolism drives immunity
Abstract
Lymphocytes must adapt to a wide array of environmental stressors as part of their normal development, during which they undergo a dramatic metabolic remodeling process. Research in this area has yielded surprising findings on the roles of diverse metabolic pathways and metabolites, which have been found to regulate lymphocyte signaling and influence differentiation, function and fate. In this review, we integrate the latest findings in the field to provide an up-to-date resource on lymphocyte metabolism.
© 2015 Buck et al.
Figures
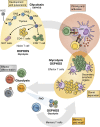
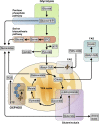
Similar articles
-
Regulation of T lymphocyte metabolism.J Immunol. 2004 Apr 15;172(8):4661-5. doi: 10.4049/jimmunol.172.8.4661. J Immunol. 2004. PMID: 15067038 Review.
-
T cell rewiring in differentiation and disease.J Immunol. 2003 Oct 1;171(7):3325-31. doi: 10.4049/jimmunol.171.7.3325. J Immunol. 2003. PMID: 14500623 Review. No abstract available.
-
T cell metabolic reprogramming and plasticity.Mol Immunol. 2015 Dec;68(2 Pt C):507-12. doi: 10.1016/j.molimm.2015.07.036. Epub 2015 Aug 12. Mol Immunol. 2015. PMID: 26277274 Free PMC article. Review.
-
Notch signalling during peripheral T-cell activation and differentiation.Nat Rev Immunol. 2007 Jan;7(1):64-75. doi: 10.1038/nri1998. Epub 2006 Dec 15. Nat Rev Immunol. 2007. PMID: 17170755 Review.
-
Unraveling the Complex Interplay Between T Cell Metabolism and Function.Annu Rev Immunol. 2018 Apr 26;36:461-488. doi: 10.1146/annurev-immunol-042617-053019. Annu Rev Immunol. 2018. PMID: 29677474 Free PMC article. Review.
Cited by
-
IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation.J Clin Invest. 2021 Jun 15;131(12):e142428. doi: 10.1172/JCI142428. J Clin Invest. 2021. PMID: 33956666 Free PMC article.
-
How Can We Engineer CAR T Cells to Overcome Resistance?Biologics. 2021 May 19;15:175-198. doi: 10.2147/BTT.S252568. eCollection 2021. Biologics. 2021. PMID: 34040345 Free PMC article. Review.
-
Polyvalent therapeutic vaccine for type 2 diabetes mellitus: Immunoinformatics approach to study co-stimulation of cytokines and GLUT1 receptors.BMC Mol Cell Biol. 2020 Jul 23;21(1):56. doi: 10.1186/s12860-020-00279-w. BMC Mol Cell Biol. 2020. PMID: 32703184 Free PMC article.
-
CD200/CD200R: Bidirectional Role in Cancer Progression and Immunotherapy.Biomedicines. 2023 Dec 16;11(12):3326. doi: 10.3390/biomedicines11123326. Biomedicines. 2023. PMID: 38137547 Free PMC article. Review.
-
High-Protein, Low-Glycaemic Meal Replacement Decreases Fasting Insulin and Inflammation Markers-A 12-Month Subanalysis of the ACOORH Trial.Nutrients. 2021 Apr 23;13(5):1433. doi: 10.3390/nu13051433. Nutrients. 2021. PMID: 33922802 Free PMC article. Clinical Trial.
References
-
- Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S., et al. . 2011. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 334:1278–1283. 10.1126/science.1211485 - DOI - PMC - PubMed
-
- Angelini G., Gardella S., Ardy M., Ciriolo M.R., Filomeni G., Di Trapani G., Clarke F., Sitia R., and Rubartelli A.. 2002. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl. Acad. Sci. USA. 99:1491–1496. 10.1073/pnas.022630299 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

