Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis
- PMID: 26258068
- PMCID: PMC4507461
- DOI: 10.3389/fonc.2015.00155
Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis
Abstract
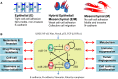
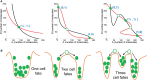
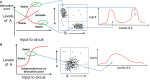
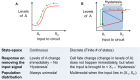
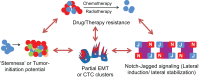
Transitions between epithelial and mesenchymal phenotypes - the epithelial to -mesenchymal transition (EMT) and its reverse the mesenchymal to epithelial transition (MET) - are hallmarks of cancer metastasis. While transitioning between the epithelial and mesenchymal phenotypes, cells can also attain a hybrid epithelial/mesenchymal (E/M) (i.e., partial or intermediate EMT) phenotype. Cells in this phenotype have mixed epithelial (e.g., adhesion) and mesenchymal (e.g., migration) properties, thereby allowing them to move collectively as clusters. If these clusters reach the bloodstream intact, they can give rise to clusters of circulating tumor cells (CTCs), as have often been seen experimentally. Here, we review the operating principles of the core regulatory network for EMT/MET that acts as a "three-way" switch giving rise to three distinct phenotypes - E, M and hybrid E/M - and present a theoretical framework that can elucidate the role of many other players in regulating epithelial plasticity. Furthermore, we highlight recent studies on partial EMT and its association with drug resistance and tumor-initiating potential; and discuss how cell-cell communication between cells in a partial EMT phenotype can enable the formation of clusters of CTCs. These clusters can be more apoptosis-resistant and have more tumor-initiating potential than singly moving CTCs with a wholly mesenchymal (complete EMT) phenotype. Also, more such clusters can be formed under inflammatory conditions that are often generated by various therapies. Finally, we discuss the multiple advantages that the partial EMT or hybrid E/M phenotype have as compared to a complete EMT phenotype and argue that these collectively migrating cells are the primary "bad actors" of metastasis.
Keywords: cancer stem cells; cancer systems biology; cell-fate decisions; intermediate EMT; partial EMT.
Figures

Similar articles
-
Stability of the hybrid epithelial/mesenchymal phenotype.Oncotarget. 2016 May 10;7(19):27067-84. doi: 10.18632/oncotarget.8166. Oncotarget. 2016. PMID: 27008704 Free PMC article.
-
OVOL guides the epithelial-hybrid-mesenchymal transition.Oncotarget. 2015 Jun 20;6(17):15436-48. doi: 10.18632/oncotarget.3623. Oncotarget. 2015. PMID: 25944618 Free PMC article.
-
NRF2 activates a partial epithelial-mesenchymal transition and is maximally present in a hybrid epithelial/mesenchymal phenotype.Integr Biol (Camb). 2019 Jun 1;11(6):251-263. doi: 10.1093/intbio/zyz021. Integr Biol (Camb). 2019. PMID: 31329868 Free PMC article.
-
Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas.Pharmacol Ther. 2019 Feb;194:161-184. doi: 10.1016/j.pharmthera.2018.09.007. Epub 2018 Sep 28. Pharmacol Ther. 2019. PMID: 30268772 Review.
-
Hybrid epithelial/mesenchymal phenotype(s): The 'fittest' for metastasis?Biochim Biophys Acta Rev Cancer. 2018 Dec;1870(2):151-157. doi: 10.1016/j.bbcan.2018.07.001. Epub 2018 Jul 8. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29997040 Review.
Cited by
-
Stem-Mesenchymal Signature Cell Genes Detected in Heterogeneous Circulating Melanoma Cells Correlate With Disease Stage in Melanoma Patients.Front Mol Biosci. 2020 May 29;7:92. doi: 10.3389/fmolb.2020.00092. eCollection 2020. Front Mol Biosci. 2020. PMID: 32548126 Free PMC article.
-
Intricate relationship between cancer stemness, metastasis, and drug resistance.MedComm (2020). 2024 Sep 21;5(10):e710. doi: 10.1002/mco2.710. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39309691 Free PMC article. Review.
-
αvβ3 Integrin induces partial EMT independent of TGF-β signaling.Commun Biol. 2021 Apr 21;4(1):490. doi: 10.1038/s42003-021-02003-6. Commun Biol. 2021. PMID: 33883697 Free PMC article.
-
Together we stand, apart we fall: how cell-to-cell contact/interplay provides resistance to ferroptosis.Cell Death Dis. 2020 Sep 23;11(9):789. doi: 10.1038/s41419-020-02994-w. Cell Death Dis. 2020. PMID: 32968052 Free PMC article. Review.
-
A possible role for epigenetic feedback regulation in the dynamics of the epithelial-mesenchymal transition (EMT).Phys Biol. 2019 Sep 6;16(6):066004. doi: 10.1088/1478-3975/ab34df. Phys Biol. 2019. PMID: 31342918 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

