The Evolution of the Secreted Regulatory Protein Progranulin
- PMID: 26248158
- PMCID: PMC4527844
- DOI: 10.1371/journal.pone.0133749
The Evolution of the Secreted Regulatory Protein Progranulin
Abstract
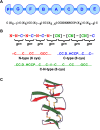
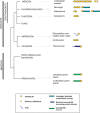
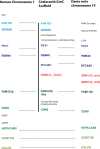
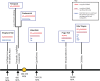
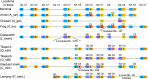
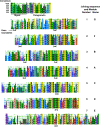
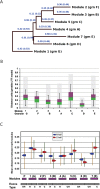
Progranulin is a secreted growth factor that is active in tumorigenesis, wound repair, and inflammation. Haploinsufficiency of the human progranulin gene, GRN, causes frontotemporal dementia. Progranulins are composed of chains of cysteine-rich granulin modules. Modules may be released from progranulin by proteolysis as 6kDa granulin polypeptides. Both intact progranulin and some of the granulin polypeptides are biologically active. The granulin module occurs in certain plant proteases and progranulins are present in early diverging metazoan clades such as the sponges, indicating their ancient evolutionary origin. There is only one Grn gene in mammalian genomes. More gene-rich Grn families occur in teleost fish with between 3 and 6 members per species including short-form Grns that have no tetrapod counterparts. Our goals are to elucidate progranulin and granulin module evolution by investigating (i): the origins of metazoan progranulins (ii): the evolutionary relationships between the single Grn of tetrapods and the multiple Grn genes of fish (iii): the evolution of granulin module architectures of vertebrate progranulins (iv): the conservation of mammalian granulin polypeptide sequences and how the conserved granulin amino acid sequences map to the known three dimensional structures of granulin modules. We report that progranulin-like proteins are present in unicellular eukaryotes that are closely related to metazoa suggesting that progranulin is among the earliest extracellular regulatory proteins still employed by multicellular animals. From the genomes of the elephant shark and coelacanth we identified contemporary representatives of a precursor for short-from Grn genes of ray-finned fish that is lost in tetrapods. In vertebrate Grns pathways of exon duplication resulted in a conserved module architecture at the amino-terminus that is frequently accompanied by an unusual pattern of tandem nearly identical module repeats near the carboxyl-terminus. Polypeptide sequence conservation of mammalian granulin modules identified potential structure-activity relationships that may be informative in designing progranulin based therapeutics.
Conflict of interest statement
Figures


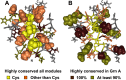

Similar articles
-
The zebrafish progranulin gene family and antisense transcripts.BMC Genomics. 2005 Nov 8;6:156. doi: 10.1186/1471-2164-6-156. BMC Genomics. 2005. PMID: 16277664 Free PMC article.
-
Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities.Protein Sci. 2008 Apr;17(4):711-24. doi: 10.1110/ps.073295308. Protein Sci. 2008. PMID: 18359860 Free PMC article.
-
Disulfide bonds and disorder in granulin-3: An unusual handshake between structural stability and plasticity.Protein Sci. 2017 Sep;26(9):1759-1772. doi: 10.1002/pro.3212. Epub 2017 Jun 22. Protein Sci. 2017. PMID: 28608407 Free PMC article.
-
Progranulin: a new avenue towards the understanding and treatment of neurodegenerative disease.Brain. 2017 Dec 1;140(12):3081-3104. doi: 10.1093/brain/awx198. Brain. 2017. PMID: 29053785 Review.
-
Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration.J Biol Chem. 2012 Sep 21;287(39):32298-306. doi: 10.1074/jbc.R112.399170. Epub 2012 Aug 2. J Biol Chem. 2012. PMID: 22859297 Free PMC article. Review.
Cited by
-
Targeting Progranulin as an Immuno-Neurology Therapeutic Approach.Int J Mol Sci. 2023 Nov 3;24(21):15946. doi: 10.3390/ijms242115946. Int J Mol Sci. 2023. PMID: 37958929 Free PMC article. Review.
-
The lysosomal function of progranulin, a guardian against neurodegeneration.Acta Neuropathol. 2018 Jul;136(1):1-17. doi: 10.1007/s00401-018-1861-8. Epub 2018 May 9. Acta Neuropathol. 2018. PMID: 29744576 Free PMC article. Review.
-
Anti-Inflammatory Targets for the Treatment of Reperfusion Injury in Stroke.Front Neurol. 2017 Sep 7;8:467. doi: 10.3389/fneur.2017.00467. eCollection 2017. Front Neurol. 2017. PMID: 28936196 Free PMC article. Review.
-
Progranulin Insufficiency Affects Lysosomal Homeostasis in Retinal Pigment Epithelium.In Vivo. 2022 Mar-Apr;36(2):610-617. doi: 10.21873/invivo.12744. In Vivo. 2022. PMID: 35241513 Free PMC article.
-
New discovery rarely runs smooth: an update on progranulin/TNFR interactions.Protein Cell. 2015 Nov;6(11):792-803. doi: 10.1007/s13238-015-0213-x. Epub 2015 Sep 25. Protein Cell. 2015. PMID: 26408020 Free PMC article. Review.
References
-
- Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(5):1715–9. Epub 1992/03/01. PubMed PMID: - PMC - PubMed
-
- Plowman GD, Green JM, Neubauer MG, Buckley SD, McDonald VL, Todaro GJ, et al. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. The Journal of biological chemistry. 1992;267(18):13073–8. Epub 1992/06/25. PubMed PMID: . - PubMed
-
- Tolkatchev D, Malik S, Vinogradova A, Wang P, Chen Z, Xu P, et al. Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein science: a publication of the Protein Society. 2008;17(4):711–24. Epub 2008/03/25. 10.1110/ps.073295308 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

