microRNA-93 promotes cell proliferation via targeting of PTEN in Osteosarcoma cells
- PMID: 26243299
- PMCID: PMC4524362
- DOI: 10.1186/s13046-015-0192-z
microRNA-93 promotes cell proliferation via targeting of PTEN in Osteosarcoma cells
Abstract
Background: Aberrant microRNA (miRNA) expression plays an essential role in osteosarcoma (OS) pathogenesis. Recent studies have shown that dysregulation of miRNA expression is associated with increased tumorigenesis and poor prognosis in several types of cancers, including OS. The aim of this study was to investigate the relevant microRNAs involved in the development of OS.
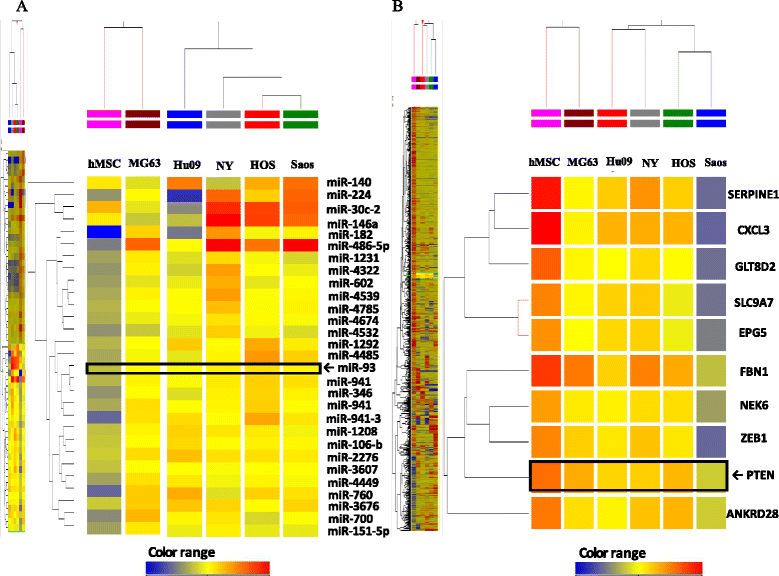
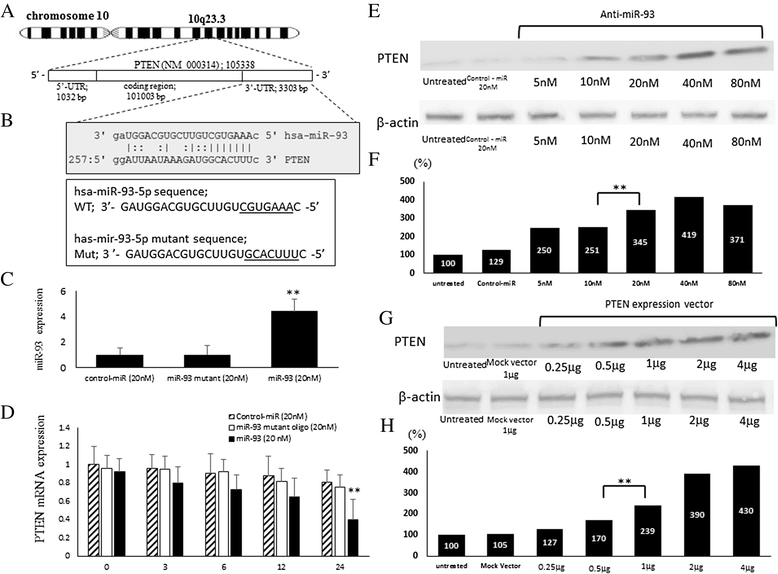
Methods: To explore possible oncogenic factors in OS, we used a microarray-based approach to profile changes in the expression of miRNAs and their target mRNAs in five OS cell lines and human mesenchymal stem cells (hMSCs). An miRNA, miR-93, was significantly up-regulated, whereas phosphatase and tensin homologue (PTEN) expression was significantly down-regulated in all tested OS cells, when compared with hMSCs.
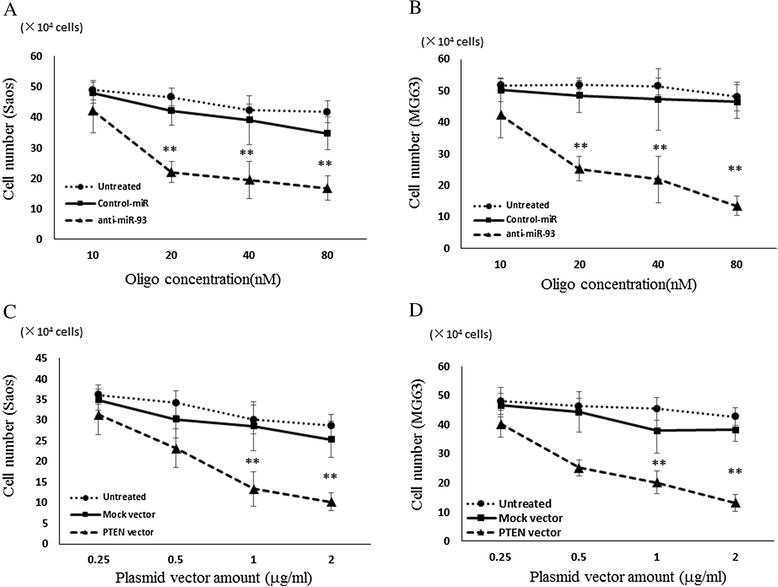
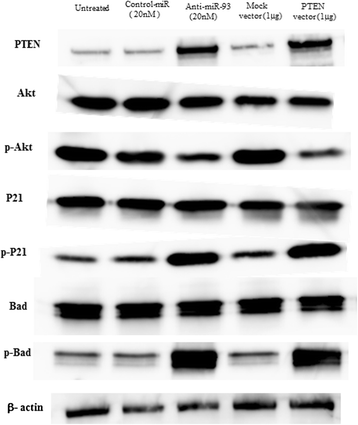
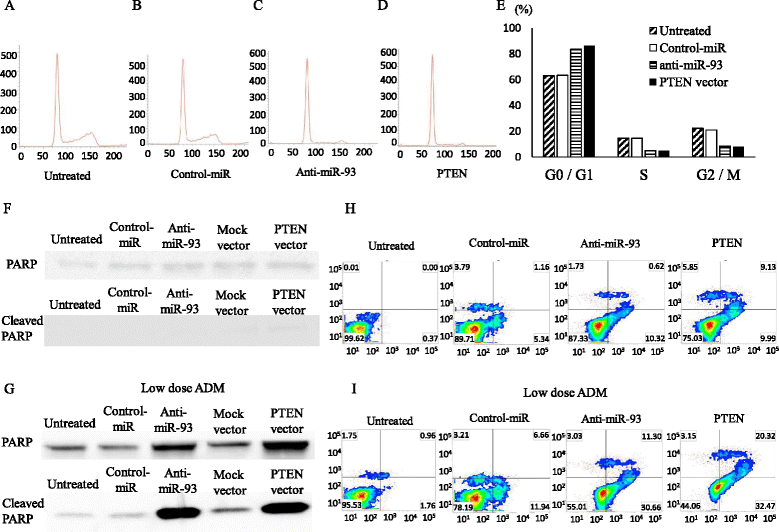
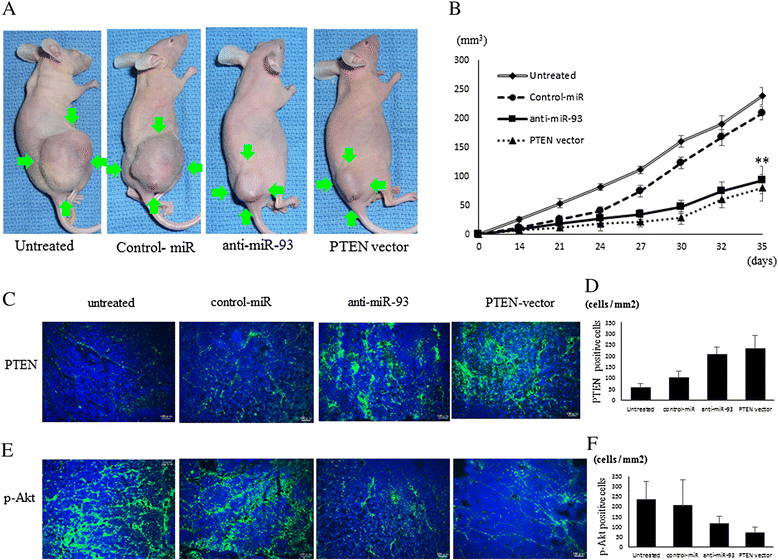
Results: When anti-miR-93 was transfected into OS cell lines, PTEN expression was greatly increased, suggesting that PTEN might be a target of miR-93 in ES cells. The expression of phosphorylated Akt protein, which is known to be inversely correlated with that of PTEN, was significantly down-regulated in anti-miR-93-transfected cells. Furthermore, transfection of anti-miR-93 inhibited the proliferation and cell cycle progression of ES cells. In addition, the down-regulation of miR-93 in these cells significantly suppressed tumor growth in vivo.
Conclusion: Ectopic expression of miR-93 decreased PTEN protein levels. Furthermore, miR-93 increased proliferation and decreased apoptosis in OS cells, whereas its silencing in these cells inhibited such carcinogenic processes. Taking these observations together, miR-93 can be seen to play a critical role in carcinogenesis through suppression of PTEN, and may serve as a therapeutic target for the treatment of OS.
Figures
Similar articles
-
MicroRNA-301a promotes cell proliferation via PTEN targeting in Ewing's sarcoma cells.Int J Oncol. 2016 Apr;48(4):1531-40. doi: 10.3892/ijo.2016.3379. Epub 2016 Feb 5. Int J Oncol. 2016. PMID: 26846737
-
miR-92a promotes tumor growth of osteosarcoma by targeting PTEN/AKT signaling pathway.Oncol Rep. 2017 Apr;37(4):2513-2521. doi: 10.3892/or.2017.5484. Epub 2017 Mar 2. Oncol Rep. 2017. PMID: 28260104
-
MicroRNA-196a overexpression promotes cell proliferation and inhibits cell apoptosis through PTEN/Akt/FOXO1 pathway.Int J Clin Exp Pathol. 2015 Mar 1;8(3):2461-72. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26045752 Free PMC article.
-
MiR-214 is an important regulator of the musculoskeletal metabolism and disease.J Cell Physiol. 2018 Jan;234(1):231-245. doi: 10.1002/jcp.26856. Epub 2018 Aug 4. J Cell Physiol. 2018. PMID: 30076721 Review.
-
Apoptosis and the target genes of microRNA-21.Chin J Cancer. 2011 Jun;30(6):371-80. doi: 10.5732/cjc.011.10132. Chin J Cancer. 2011. PMID: 21627859 Free PMC article. Review.
Cited by
-
LncRNA PLAC 2 Is Downregulated in Osteosarcoma and Regulates Cancer Cell Proliferation Through miR-93.Cancer Manag Res. 2020 May 20;12:3623-3629. doi: 10.2147/CMAR.S238295. eCollection 2020. Cancer Manag Res. 2020. PMID: 32547199 Free PMC article.
-
MicroRNA-885-5p promotes osteosarcoma proliferation and migration by downregulation of cell division cycle protein 73 homolog expression.Oncol Lett. 2019 Feb;17(2):1565-1572. doi: 10.3892/ol.2018.9802. Epub 2018 Dec 6. Oncol Lett. 2019. PMID: 30675214 Free PMC article.
-
miRNA signatures in childhood sarcomas and their clinical implications.Clin Transl Oncol. 2019 Dec;21(12):1583-1623. doi: 10.1007/s12094-019-02104-z. Epub 2019 Apr 4. Clin Transl Oncol. 2019. PMID: 30949930 Review.
-
MicroRNAs for osteosarcoma in the mouse: a meta-analysis.Oncotarget. 2016 Dec 20;7(51):85650-85674. doi: 10.18632/oncotarget.13333. Oncotarget. 2016. PMID: 27852052 Free PMC article. Review.
-
MicroRNA-93 promotes cell proliferation by directly targeting P21 in osteosarcoma cells.Exp Ther Med. 2017 May;13(5):2003-2011. doi: 10.3892/etm.2017.4204. Epub 2017 Mar 8. Exp Ther Med. 2017. Retraction in: Exp Ther Med. 2024 Jan 15;27(3):101. doi: 10.3892/etm.2024.12389. PMID: 28565800 Free PMC article. Retracted.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

