Circadian Rhythms in Rho1 Activity Regulate Neuronal Plasticity and Network Hierarchy
- PMID: 26234154
- PMCID: PMC4537806
- DOI: 10.1016/j.cell.2015.07.010
Circadian Rhythms in Rho1 Activity Regulate Neuronal Plasticity and Network Hierarchy
Abstract
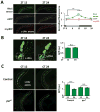
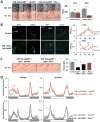
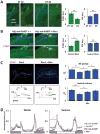
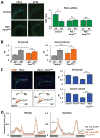
Neuronal plasticity helps animals learn from their environment. However, it is challenging to link specific changes in defined neurons to altered behavior. Here, we focus on circadian rhythms in the structure of the principal s-LNv clock neurons in Drosophila. By quantifying neuronal architecture, we observed that s-LNv structural plasticity changes the amount of axonal material in addition to cycles of fasciculation and defasciculation. We found that this is controlled by rhythmic Rho1 activity that retracts s-LNv axonal termini by increasing myosin phosphorylation and simultaneously changes the balance of pre-synaptic and dendritic markers. This plasticity is required to change clock network hierarchy and allow seasonal adaptation. Rhythms in Rho1 activity are controlled by clock-regulated transcription of Puratrophin-1-like (Pura), a Rho1 GEF. Since spinocerebellar ataxia is associated with mutations in human Puratrophin-1, our data support the idea that defective actin-related plasticity underlies this ataxia.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Neurons Rho to Get in Shape for the Day.Cell. 2015 Aug 13;162(4):699-700. doi: 10.1016/j.cell.2015.07.044. Cell. 2015. PMID: 26276623
Similar articles
-
The Pebble/Rho1/Anillin pathway controls polyploidization and axonal wrapping activity in the glial cells of the Drosophila eye.Dev Biol. 2021 May;473:90-96. doi: 10.1016/j.ydbio.2021.02.002. Epub 2021 Feb 11. Dev Biol. 2021. PMID: 33581137
-
Neurons Rho to Get in Shape for the Day.Cell. 2015 Aug 13;162(4):699-700. doi: 10.1016/j.cell.2015.07.044. Cell. 2015. PMID: 26276623
-
Circadian plasticity: from structure to behavior.Int Rev Neurobiol. 2011;99:107-38. doi: 10.1016/B978-0-12-387003-2.00005-7. Int Rev Neurobiol. 2011. PMID: 21906538 Review.
-
The transcription factor Mef2 links the Drosophila core clock to Fas2, neuronal morphology, and circadian behavior.Neuron. 2013 Jul 24;79(2):281-92. doi: 10.1016/j.neuron.2013.05.015. Neuron. 2013. PMID: 23889933 Free PMC article.
-
Circadian Plasticity in the Brain of Insects and Rodents.Front Neural Circuits. 2018 May 2;12:32. doi: 10.3389/fncir.2018.00032. eCollection 2018. Front Neural Circuits. 2018. PMID: 29770112 Free PMC article. Review.
Cited by
-
Genetic regulation of central synapse formation and organization in Drosophila melanogaster.Genetics. 2022 Jul 4;221(3):iyac078. doi: 10.1093/genetics/iyac078. Genetics. 2022. PMID: 35652253 Free PMC article. Review.
-
CLOCK and BMAL1 stabilize and activate RHOA to promote F-actin formation in cancer cells.Exp Mol Med. 2018 Oct 4;50(10):1-15. doi: 10.1038/s12276-018-0156-4. Exp Mol Med. 2018. PMID: 30287810 Free PMC article.
-
CaveCrawler: an interactive analysis suite for cavefish bioinformatics.G3 (Bethesda). 2022 Jul 29;12(8):jkac132. doi: 10.1093/g3journal/jkac132. G3 (Bethesda). 2022. PMID: 35708643 Free PMC article.
-
Circuit formation in the adult brain.Eur J Neurosci. 2022 Aug;56(3):4187-4213. doi: 10.1111/ejn.15742. Epub 2022 Jul 1. Eur J Neurosci. 2022. PMID: 35724981 Free PMC article. Review.
-
Glial Regulation of Circuit Wiring, Firing, and Expiring in the Drosophila Central Nervous System.Cold Spring Harb Perspect Biol. 2024 Dec 2;16(12):a041347. doi: 10.1101/cshperspect.a041347. Cold Spring Harb Perspect Biol. 2024. PMID: 38565270 Review.
References
-
- Amino T, Ishikawa K, Toru S, Ishiguro T, Sato N, Tsunemi T, Murata M, Kobayashi K, Inazawa J, Toda T, et al. Redefining the disease locus of 16q22.1-linked autosomal dominant cerebellar ataxia. J Hum Genet. 2007;52:643–649. - PubMed
-
- Becquet D, Girardet C, Guillaumond F, Francois-Bellan AM, Bosler O. Ultrastructural plasticity in the rat suprachiasmatic nucleus. Possible involvement in clock entrainment. Glia. 2008;56:294–305. - PubMed
-
- Billuart P, Winter CG, Maresh A, Zhao X, Luo L. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell. 2001;107:195–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

