The role of sirtuins in cardiac disease
- PMID: 26232232
- PMCID: PMC4666968
- DOI: 10.1152/ajpheart.00053.2015
The role of sirtuins in cardiac disease
Abstract
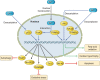
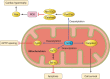
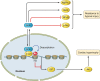
Modification of histones is one of the important mechanisms of epigenetics, in which genetic control is determined by factors other than an individual's DNA sequence. Sirtuin family proteins, which are class III histone deacetylases, were originally identified as gene silencers that affect the mating type of yeast, leading to the name "silent mating-type information regulation 2" (SIR2). They are characterized by their requirement of nicotinamide adenine dinucleotide for their enzyme activity, unlike other classes of histone deacetylases. Sirtuins have been traditionally linked to longevity and the beneficial effects of calorie restriction and DNA damage repair. Recently, sirtuins have been shown to be involved in a wide range of physiological and pathological processes, including aging, energy responses to low calorie availability, and stress resistance, as well as apoptosis and inflammation. Sirtuins can also regulate mitochondrial biogenesis and circadian clocks. Seven sirtuin family proteins (Sirt1-7) have been identified as mammalian SIR2 orthologs, localized in different subcellular compartments, namely, the cytoplasm (Sirt1, 2), the mitochondria (Sirt3, 4, 5), and the nucleus (Sirt1, 2, 6, 7). Sirt1 is evolutionarily close to yeast SIR2 and has been the most intensively investigated in the cardiovascular system. Endogenous Sirt1 plays a pivotal role in mediating the cell death/survival process and has been implicated in the pathogenesis of cardiovascular disease. Downregulation of Sirt2 is protective against ischemic-reperfusion injury. Increased Sirt3 expression has been shown to correlate with longevity in humans. In addition, Sirt3 protects cardiomyocytes from aging and oxidative stress and suppresses cardiac hypertrophy. Sirt6 has also recently been demonstrated to attenuate cardiac hypertrophy, and Sirt7 is known to regulate apoptosis and stress responses in the heart. On the other hand, the roles of Sirt4 and Sirt5 in the heart remain largely uncharacterized.
Keywords: FoxO; cell death/survival; longevity; sirtuin-activating compounds.
Copyright © 2015 the American Physiological Society.
Figures
Similar articles
-
Versatile role of sirtuins in metabolic disorders: From modulation of mitochondrial function to therapeutic interventions.J Biochem Mol Toxicol. 2022 Jul;36(7):e23047. doi: 10.1002/jbt.23047. Epub 2022 Mar 17. J Biochem Mol Toxicol. 2022. PMID: 35297126 Review.
-
Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes.Circ Res. 2004 Nov 12;95(10):971-80. doi: 10.1161/01.RES.0000147557.75257.ff. Epub 2004 Oct 14. Circ Res. 2004. PMID: 15486319
-
Sirtuin family in autoimmune diseases.Front Immunol. 2023 Jul 6;14:1186231. doi: 10.3389/fimmu.2023.1186231. eCollection 2023. Front Immunol. 2023. PMID: 37483618 Free PMC article. Review.
-
Sirtuins and renal diseases: relationship with aging and diabetic nephropathy.Clin Sci (Lond). 2013 Feb;124(3):153-64. doi: 10.1042/CS20120190. Clin Sci (Lond). 2013. PMID: 23075334 Free PMC article. Review.
-
Screening Analysis of Sirtuins Family Expression on Anti-Inflammation of Resveratrol in Endothelial Cells.Med Sci Monit. 2019 Jun 3;25:4137-4148. doi: 10.12659/MSM.913240. Med Sci Monit. 2019. PMID: 31158122 Free PMC article.
Cited by
-
In Patients with Coronary Artery Disease and Type 2 Diabetes, SIRT1 Expression in Circulating Mononuclear Cells Is Associated with Levels of Inflammatory Cytokines but Not with Coronary Lesions.Biomed Res Int. 2016;2016:8734827. doi: 10.1155/2016/8734827. Epub 2016 Mar 31. Biomed Res Int. 2016. PMID: 27123454 Free PMC article.
-
Bakuchiol Alleviates Hyperglycemia-Induced Diabetic Cardiomyopathy by Reducing Myocardial Oxidative Stress via Activating the SIRT1/Nrf2 Signaling Pathway.Oxid Med Cell Longev. 2020 Sep 30;2020:3732718. doi: 10.1155/2020/3732718. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33062139 Free PMC article.
-
The Role of Posttranslational Modification and Mitochondrial Quality Control in Cardiovascular Diseases.Oxid Med Cell Longev. 2021 Feb 18;2021:6635836. doi: 10.1155/2021/6635836. eCollection 2021. Oxid Med Cell Longev. 2021. Retraction in: Oxid Med Cell Longev. 2023 Oct 11;2023:9821720. doi: 10.1155/2023/9821720 PMID: 33680284 Free PMC article. Retracted. Review.
-
Molecular Imaging of Sirtuin1 Expression-Activity in Rat Brain Using Positron-Emission Tomography-Magnetic-Resonance Imaging with [18F]-2-Fluorobenzoylaminohexanoicanilide.J Med Chem. 2018 Aug 23;61(16):7116-7130. doi: 10.1021/acs.jmedchem.8b00253. Epub 2018 Aug 13. J Med Chem. 2018. PMID: 30052441 Free PMC article.
-
Sex differences in the aging human heart: decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense.Aging (Albany NY). 2019 Apr 8;11(7):1918-1933. doi: 10.18632/aging.101881. Aging (Albany NY). 2019. PMID: 30964749 Free PMC article.
References
-
- Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 133: 978–993, 2008. - PubMed
-
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

