Genes associated with Parkinson's disease: regulation of autophagy and beyond
- PMID: 26223426
- PMCID: PMC5834228
- DOI: 10.1111/jnc.13266
Genes associated with Parkinson's disease: regulation of autophagy and beyond
Abstract
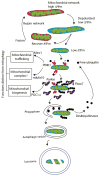
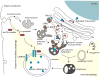
Substantial progress has been made in the genetic basis of Parkinson's disease (PD). In particular, by identifying genes that segregate with inherited PD or show robust association with sporadic disease, and by showing the same genes are found on both lists, we have generated an outline of the cause of this condition. Here, we will discuss what those genes tell us about the underlying biology of PD. We specifically discuss the relationships between protein products of PD genes and show that common links include regulation of the autophagy-lysosome system, an important way by which cells recycle proteins and organelles. We also discuss whether all PD genes should be considered to be in the same pathway and propose that in some cases the relationships are closer, whereas in other cases the interactions are more distant and might be considered separate. Beilina and Cookson review the links between genes for Parkinson's disease (red) and the autophagy-lysosomal system. They propose the hypothesis that many of the known PD genes can be assigned to pathways that affect (I) turnover of mitochondria via mitophagy (II) turnover of several vesicular structures via macroautophagy or chaperone-mediated autophagy or (III) general lysosome function. This article is part of a special issue on Parkinson disease.
Keywords: Parkinson's disease; chaperone-mediated autophagy; genetics; lysosomes; mitophagy; protein complexes.
Published 2015. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Genetic causes of Parkinson's disease and their links to autophagy regulation.Parkinsonism Relat Disord. 2014 Jan;20 Suppl 1:S154-7. doi: 10.1016/S1353-8020(13)70037-3. Parkinsonism Relat Disord. 2014. PMID: 24262170 Review.
-
Autophagy and Alpha-Synuclein: Relevance to Parkinson's Disease and Related Synucleopathies.Mov Disord. 2016 Feb;31(2):178-92. doi: 10.1002/mds.26477. Epub 2016 Jan 27. Mov Disord. 2016. PMID: 26813776 Review.
-
Genetic perspective on the role of the autophagy-lysosome pathway in Parkinson disease.Autophagy. 2015;11(9):1443-57. doi: 10.1080/15548627.2015.1067364. Autophagy. 2015. PMID: 26207393 Free PMC article. Review.
-
Autophagy lysosomal pathway dysfunction in Parkinson's disease; evidence from human genetics.Parkinsonism Relat Disord. 2020 Apr;73:60-71. doi: 10.1016/j.parkreldis.2019.11.015. Epub 2019 Nov 17. Parkinsonism Relat Disord. 2020. PMID: 31761667 Review.
-
Crosstalk of organelles in Parkinson's disease - MiT family transcription factors as central players in signaling pathways connecting mitochondria and lysosomes.Mol Neurodegener. 2022 Jul 16;17(1):50. doi: 10.1186/s13024-022-00555-7. Mol Neurodegener. 2022. PMID: 35842725 Free PMC article. Review.
Cited by
-
Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):156-178. doi: 10.1111/jnc.13572. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26865375 Free PMC article. Review.
-
Predictive Big Data Analytics: A Study of Parkinson's Disease Using Large, Complex, Heterogeneous, Incongruent, Multi-Source and Incomplete Observations.PLoS One. 2016 Aug 5;11(8):e0157077. doi: 10.1371/journal.pone.0157077. eCollection 2016. PLoS One. 2016. PMID: 27494614 Free PMC article.
-
The SATB1-MIR22-GBA axis mediates glucocerebroside accumulation inducing a cellular senescence-like phenotype in dopaminergic neurons.Aging Cell. 2024 Apr;23(4):e14077. doi: 10.1111/acel.14077. Epub 2024 Feb 1. Aging Cell. 2024. PMID: 38303548 Free PMC article.
-
Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease.Cell. 2016 Dec 1;167(6):1469-1480.e12. doi: 10.1016/j.cell.2016.11.018. Cell. 2016. PMID: 27912057 Free PMC article.
-
Parkinson's Disease and Photobiomodulation: Potential for Treatment.J Pers Med. 2024 Jan 19;14(1):112. doi: 10.3390/jpm14010112. J Pers Med. 2024. PMID: 38276234 Free PMC article. Review.
References
-
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AHV. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–1472. - PubMed
-
- Ando M, Funayama M, Li Y, et al. VPS35 mutation in Japanese patients with typical Parkinson’s disease. Mov Disord. 2012;27:1413–1417. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

