Ribavirin Concentrations Do Not Predict Sustained Virological Response in HIV/HCV-Coinfected Patients Treated with Ribavirin and Pegylated Interferon in the Swiss HIV Cohort Study
- PMID: 26218843
- PMCID: PMC4517877
- DOI: 10.1371/journal.pone.0133879
Ribavirin Concentrations Do Not Predict Sustained Virological Response in HIV/HCV-Coinfected Patients Treated with Ribavirin and Pegylated Interferon in the Swiss HIV Cohort Study
Abstract
Background: Ribavirin (RBV) is an essential component of most current hepatitis C (HCV) treatment regimens and still standard of care in the combination with pegylated interferon (pegIFN) to treat chronic HCV in resource limited settings. Study results in HIV/HCV-coinfected patients are contradicting as to whether RBV concentration correlates with sustained virological response (SVR).
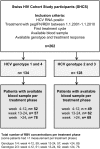
Methods: We included 262 HCV treatment naïve HIV/HCV-coinfected Swiss HIV Cohort Study (SHCS) participants treated with RBV and pegIFN between 01.01.2001-01.01.2010, 134 with HCV genotype (GT) 1/4, and 128 with GT 2/3 infections. RBV levels were measured retrospectively in stored plasma samples obtained between HCV treatment week 4 and end of therapy. Uni- and multivariable logistic regression analyses were used to evaluate the association between RBV concentration and SVR in GT 1/4 and GT 2/3 infections. The analyses were repeated stratified by treatment phase (week 4-12, 13-24, >24) and IL28B genotype (CC versus CT/TT).
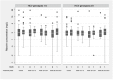
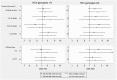
Results: SVR rates were 35.1% in GT 1/4 and 70.3% in GT 2/3 infections. Overall, median RBV concentration was 2.0 mg/L in GT 1/4, and 1.9 mg/L in GT 2/3, and did not change significantly across treatment phases. Patients with SVR had similar RBV concentrations compared to patients without SVR in both HCV genotype groups. SVR was not associated with RBV levels ≥2.0 mg/L (GT 1/4, OR 1.19 [0.5-2.86]; GT 2/3, 1.94 [0.78-4.80]) and ≥2.5 mg/L (GT 1/4, 1.56 [0.64-3.84]; GT 2/3 2.72 [0.85-8.73]), regardless of treatment phase, and IL28B genotype.
Conclusion: In HIV/HCV-coinfected patients treated with pegIFN/RBV, therapeutic drug monitoring of RBV concentrations does not enhance the chance of HCV cure, regardless of HCV genotype, treatment phase and IL28B genotype.
Conflict of interest statement
Figures
Similar articles
-
Relative impact of ribavirin monitoring and HIV coinfection on sustained virological response in patients with chronic hepatitis C.Antivir Ther. 2011;16(8):1317-26. doi: 10.3851/IMP1920. Antivir Ther. 2011. PMID: 22155913
-
Effect of aging, glucose level, and HIV viral load on response to treatment with pegylated interferon plus ribavirin in HIV/HCV co-infected women.J Womens Health (Larchmt). 2015 Feb;24(2):159-64. doi: 10.1089/jwh.2014.4796. Epub 2015 Feb 3. J Womens Health (Larchmt). 2015. PMID: 25682817
-
Efficacy and safety of daclatasvir plus pegylated-interferon alfa 2a and ribavirin in previously untreated HCV subjects coinfected with HIV and HCV genotype-1: a Phase III, open-label study.Hepatol Int. 2017 Mar;11(2):188-198. doi: 10.1007/s12072-017-9788-z. Epub 2017 Feb 16. Hepatol Int. 2017. PMID: 28210927 Clinical Trial.
-
Simeprevir with pegylated interferon alfa 2a plus ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a meta-analysis and historical comparison.BMC Infect Dis. 2016 Jan 11;16:10. doi: 10.1186/s12879-015-1311-3. BMC Infect Dis. 2016. PMID: 26753774 Free PMC article. Review.
-
Influence of Host and Viral Factors on Patients with Chronic Hepatitis C Virus Genotype 6 Treated with Pegylated Interferon and Ribavirin: A Systematic Review and Meta-Analysis.Intervirology. 2015;58(6):373-81. doi: 10.1159/000444366. Epub 2016 Mar 25. Intervirology. 2015. PMID: 27010195 Review.
Cited by
-
Sofosbuvir-based therapies in genotype 2 hepatitis C virus cirrhosis: A real-life experience with focus on ribavirin dose.Pharmacol Res Perspect. 2021 Aug;9(4):e00811. doi: 10.1002/prp2.811. Pharmacol Res Perspect. 2021. PMID: 34152088 Free PMC article.
-
Influence of Ribavirin Serum Levels on Outcome of Antiviral Treatment and Anemia in Hepatitis C Virus Infection.PLoS One. 2016 Jul 7;11(7):e0158512. doi: 10.1371/journal.pone.0158512. eCollection 2016. PLoS One. 2016. PMID: 27388623 Free PMC article.
References
-
- Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection WHO Guidelines Approved by the Guidelines Review Committee. Geneva: 2014. - PubMed
-
- Russmann S, Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Current medicinal chemistry. 2006;13(27):3351–7. - PubMed
-
- Jen JF, Glue P, Gupta S, Zambas D, Hajian G. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Therapeutic drug monitoring. 2000;22(5):555–65. - PubMed
-
- Slavenburg S, Huntjens-Fleuren HW, Dofferhoff TS, Richter C, Koopmans PP, Verwey-Van Wissen CP, et al. Ribavirin plasma concentration measurements in patients with hepatitis C: early ribavirin concentrations predict steady-state concentrations. Therapeutic drug monitoring. 2011;33(1):40–4. 10.1097/FTD.0b013e318205f892 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

