First international external quality assessment of molecular diagnostics for Mers-CoV
- PMID: 26209385
- PMCID: PMC7106520
- DOI: 10.1016/j.jcv.2015.05.022
First international external quality assessment of molecular diagnostics for Mers-CoV
Abstract
Background: Since the discovery of Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, diagnostic protocols were quickly published and deployed globally.
Objectives: We set out to assess the quality of MERS-CoV molecular diagnostics worldwide.
Study design: Both sensitivity and specificity were assessed using 12 samples containing different viral loads of MERS-CoV or common coronaviruses (OC43, 229E, NL63, HKU1).
Results: The panel was sent to more than 106 participants, of which 99 laboratories from 6 continents returned 189 panel results.Scores ranged from 100% (84 laboratories) to 33% (1 laboratory). 15% of respondents reported quantitative results, 61% semi-quantitative (Ct-values or time to positivity) and 24% reported qualitative results. The major specific technique used was real-time RT-PCR using the WHO recommended targets upE, ORF1a and ORF1b. The evaluation confirmed that RT-PCRs targeting the ORF1b are less sensitive, and therefore not advised for primary diagnostics.
Conclusions: The first external quality assessment MERS-CoV panel gives a good insight in molecular diagnostic techniques and their performances for sensitive and specific detection of MERS-CoV RNA globally. Overall, all laboratories were capable of detecting MERS-CoV with some differences in sensitivity. The observation that 8% of laboratories reported false MERS-CoV positive single assay results shows room for improvement, and the importance of using confirmatory targets.
Keywords: Diagnosis; EQA; MERS-CoV; Molecular; QPCR; Quality control; Real-time RT-PCR; Viral load.
Copyright © 2015 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
External Quality Assessment of MERS-CoV Molecular Diagnostics During the 2015 Korean Outbreak.Ann Lab Med. 2016 May;36(3):230-4. doi: 10.3343/alm.2016.36.3.230. Ann Lab Med. 2016. PMID: 26915611 Free PMC article.
-
Performance Evaluation of the PowerChek MERS (upE & ORF1a) Real-Time PCR Kit for the Detection of Middle East Respiratory Syndrome Coronavirus RNA.Ann Lab Med. 2017 Nov;37(6):494-498. doi: 10.3343/alm.2017.37.6.494. Ann Lab Med. 2017. PMID: 28840986 Free PMC article.
-
External quality assessment for the molecular detection of MERS-CoV in China.J Clin Virol. 2016 Feb;75:5-9. doi: 10.1016/j.jcv.2015.12.001. Epub 2015 Dec 9. J Clin Virol. 2016. PMID: 26702992 Free PMC article.
-
MERS-CoV diagnosis: An update.J Infect Public Health. 2016 May-Jun;9(3):216-9. doi: 10.1016/j.jiph.2016.04.005. Epub 2016 Apr 20. J Infect Public Health. 2016. PMID: 27106390 Free PMC article. Review.
-
Korean Society for Laboratory Medicine Practice Guidelines for the Molecular Diagnosis of Middle East Respiratory Syndrome During an Outbreak in Korea in 2015.Ann Lab Med. 2016 May;36(3):203-8. doi: 10.3343/alm.2016.36.3.203. Ann Lab Med. 2016. PMID: 26915607 Free PMC article. Review.
Cited by
-
Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR.Euro Surveill. 2020 Jan;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Euro Surveill. 2020. PMID: 31992387 Free PMC article.
-
The role of laboratory diagnostics in emerging viral infections: the example of the Middle East respiratory syndrome epidemic.J Microbiol. 2017 Mar;55(3):172-182. doi: 10.1007/s12275-017-7026-y. Epub 2017 Feb 28. J Microbiol. 2017. PMID: 28243939 Free PMC article. Review.
-
Variable Sensitivity of SARS-CoV-2 Molecular Detection in European Expert Laboratories: External Quality Assessment, June and July 2020.J Clin Microbiol. 2021 Feb 18;59(3):e02676-20. doi: 10.1128/JCM.02676-20. Print 2021 Feb 18. J Clin Microbiol. 2021. PMID: 33298612 Free PMC article.
-
Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity.Sci Rep. 2016 May 5;6:25359. doi: 10.1038/srep25359. Sci Rep. 2016. PMID: 27146253 Free PMC article.
-
Proficiency testing for the detection of Middle East respiratory syndrome coronavirus demonstrates global capacity to detect Middle East respiratory syndrome coronavirus.J Med Virol. 2018 Dec;90(12):1827-1833. doi: 10.1002/jmv.25266. Epub 2018 Aug 27. J Med Virol. 2018. PMID: 30016543 Free PMC article.
References
-
- Zaki A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. - PubMed
-
- Corman V.M. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Eurosurveillance. 2012;17(39) pii=20285. - PubMed
-
- Corman V.M. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Eurosurveillance. 2012;17(49) pii=20334. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

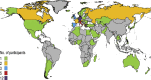
 n = 1;
n = 1;  n =…
n =…  n = 3;
n = 3;  n = 5;
n = 5;  n = 12.
n = 12.