Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein
- PMID: 26198711
- PMCID: PMC4700074
- DOI: 10.1007/s00018-015-1988-x
Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein
Abstract
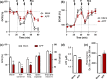
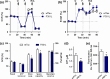
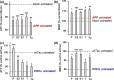
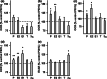
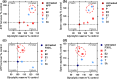
Alzheimer's disease (AD) is an age-related neurodegenerative disease marked by a progressive cognitive decline. Metabolic impairments are common hallmarks of AD, and amyloid-β (Aβ) peptide and hyperphosphorylated tau protein--the two foremost histopathological signs of AD--have been implicated in mitochondrial dysfunction. Neurosteroids have recently shown promise in alleviating cognitive and neuronal sequelae of AD. The present study evaluates the impact of neurosteroids belonging to the sex hormone family (progesterone, estradiol, estrone, testosterone, 3α-androstanediol) on mitochondrial dysfunction in cellular models of AD: human neuroblastoma cells (SH-SY5Y) stably transfected with constructs encoding (1) the human amyloid precursor protein (APP) resulting in overexpression of APP and Aβ, (2) wild-type tau (wtTau), and (3) mutant tau (P301L), that induces abnormal tau hyperphosphorylation. We show that while APP and P301L cells both display a drop in ATP levels, they present distinct mitochondrial impairments with regard to their bioenergetic profiles. The P301L cells presented a decreased maximal respiration and spare respiratory capacity, while APP cells exhibited, in addition, a decrease in basal respiration, ATP turnover, and glycolytic reserve. All neurosteroids showed beneficial effects on ATP production and mitochondrial membrane potential in APP/Aβ overexpressing cells while only progesterone and estradiol increased ATP levels in mutant tau cells. Of note, testosterone was more efficient in alleviating Aβ-induced mitochondrial deficits, while progesterone and estrogen were the most effective neurosteroids in our model of AD-related tauopathy. Our findings lend further support to the neuroprotective effects of neurosteroids in AD and may open new avenues for the development of gender-specific therapeutic approaches in AD.
Keywords: Amyloid-β peptide; Bioenergetics; Mitochondria; Neurosteroids; Tau protein.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
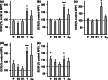
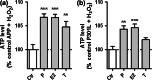
Similar articles
-
Link between the unfolded protein response and dysregulation of mitochondrial bioenergetics in Alzheimer's disease.Cell Mol Life Sci. 2019 Apr;76(7):1419-1431. doi: 10.1007/s00018-019-03009-4. Epub 2019 Jan 25. Cell Mol Life Sci. 2019. PMID: 30683981 Free PMC article.
-
Modulation of neurosteroid production in human neuroblastoma cells by Alzheimer's disease key proteins.J Neurobiol. 2006 Jul;66(8):868-81. doi: 10.1002/neu.20267. J Neurobiol. 2006. PMID: 16673391
-
Modeling Alzheimer's disease in mouse without mutant protein overexpression: cooperative and independent effects of Aβ and tau.PLoS One. 2013 Nov 20;8(11):e80706. doi: 10.1371/journal.pone.0080706. eCollection 2013. PLoS One. 2013. PMID: 24278307 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease.Cells. 2019 May 22;8(5):488. doi: 10.3390/cells8050488. Cells. 2019. PMID: 31121890 Free PMC article. Review.
Cited by
-
Aromatase Expression in the Hippocampus of AD Patients and 5xFAD Mice.Neural Plast. 2016;2016:9802086. doi: 10.1155/2016/9802086. Epub 2016 May 19. Neural Plast. 2016. PMID: 27298742 Free PMC article.
-
TSPO Ligands Boost Mitochondrial Function and Pregnenolone Synthesis.J Alzheimers Dis. 2019;72(4):1045-1058. doi: 10.3233/JAD-190127. J Alzheimers Dis. 2019. PMID: 31256132 Free PMC article.
-
Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes.Front Aging Neurosci. 2022 Jun 30;14:931536. doi: 10.3389/fnagi.2022.931536. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35847660 Free PMC article. Review.
-
Sex-Stratified Single-Cell RNA-Seq Analysis Identifies Sex-Specific and Cell Type-Specific Transcriptional Responses in Alzheimer's Disease Across Two Brain Regions.Mol Neurobiol. 2022 Jan;59(1):276-293. doi: 10.1007/s12035-021-02591-8. Epub 2021 Oct 20. Mol Neurobiol. 2022. PMID: 34669146 Free PMC article.
-
Honeybush Extracts (Cyclopia spp.) Rescue Mitochondrial Functions and Bioenergetics against Oxidative Injury.Oxid Med Cell Longev. 2020 Aug 7;2020:1948602. doi: 10.1155/2020/1948602. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32831989 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

