Galeterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivo
- PMID: 26196320
- PMCID: PMC4695001
- DOI: 10.18632/oncotarget.4578
Galeterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivo
Abstract
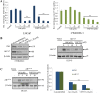
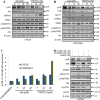
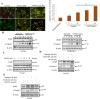
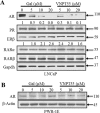
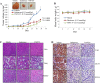
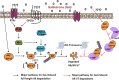
Galeterone (Gal) is a first-in-class multi-target oral small molecule that will soon enter pivotal phase III clinical trials in castration resistant prostate cancer (CRPC) patients. Gal disrupts androgen receptor (AR) signaling via inhibition of CYP17, AR antagonism and AR degradation. Resistance to current therapy is attributed to up-regulation of full-length AR (fAR), splice variants AR (AR-Vs) and AR mutations. The effects of gal and VNPT55 were analyzed on f-AR and AR-Vs (AR-V7/ARv567es) in LNCaP, CWR22Rv1 and DU145 (transfected with AR-Vs) human PC cells in vitro and CRPC tumor xenografts. Galeterone/VNPT55 decreased fAR/AR-V7 mRNA levels and implicates Mdm2/CHIP enhanced ubiquitination of posttranslational modified receptors, targeting them for proteasomal degradation. Gal and VNPT55 also induced significant apoptosis in PC cells via increased Bax/Bcl2 ratio, cytochrome-c release with concomitant cleavage of caspase 3 and PARP. More importantly, gal and VNPT55 exhibited strong in vivo anti-CRPC activities, with no apparent host toxicities. This study demonstrate that gal and VNPT55 utilize cell-based mechanisms to deplete both fAR and AR-Vs. Importantly, the preclinical activity profiles, including profound apoptotic induction and inhibition of CRPC xenografts suggest that these agents offer considerable promise as new therapeutics for patients with CRPC and those resistant to current therapy.
Keywords: androgen receptors (AR/AR-V7); galeterone (gal); gal’s analog VNPT55; mechanisms of AR/AR-V7 degradation; prostate cancer.
Conflict of interest statement
Vincent C. O. Njar is the lead inventor of galeterone (VN/124-1 or TOK-001) and VNPT55, patents and technologies thereof owned by the University of Maryland, Baltimore, and licensed to Tokai Pharmaceuticals, Inc. Puranik Purushottamachar and Andrew K. Kwegyir-Afful are co-inventors of VNPT55 and related compounds. A patent application to protect VNPT55 and related novel compounds has been filed. The other authors declare no potential conflict of interest.
Figures





Similar articles
-
Salinization Dramatically Enhance the Anti-Prostate Cancer Efficacies of AR/AR-V7 and Mnk1/2 Molecular Glue Degraders, Galeterone and VNPP433-3β Which Outperform Docetaxel and Enzalutamide in CRPC CWR22Rv1 Xenograft Mouse Model.Bioorg Chem. 2023 Oct;139:106700. doi: 10.1016/j.bioorg.2023.106700. Epub 2023 Jun 25. Bioorg Chem. 2023. PMID: 37392559 Free PMC article.
-
Galeterone and VNPT55 disrupt Mnk-eIF4E to inhibit prostate cancer cell migration and invasion.FEBS J. 2016 Nov;283(21):3898-3918. doi: 10.1111/febs.13895. Epub 2016 Oct 1. FEBS J. 2016. PMID: 27618366 Free PMC article.
-
Androgen Receptor Modulation Optimized for Response-Splice Variant: A Phase 3, Randomized Trial of Galeterone Versus Enzalutamide in Androgen Receptor Splice Variant-7-expressing Metastatic Castration-resistant Prostate Cancer.Eur Urol. 2019 Dec;76(6):843-851. doi: 10.1016/j.eururo.2019.08.034. Epub 2019 Sep 18. Eur Urol. 2019. PMID: 31542304 Clinical Trial.
-
Galeterone for the treatment of advanced prostate cancer: the evidence to date.Drug Des Devel Ther. 2016 Jul 15;10:2289-97. doi: 10.2147/DDDT.S93941. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27486306 Free PMC article. Review.
-
Role of androgen receptor splice variants, their clinical relevance and treatment options.World J Urol. 2020 Mar;38(3):647-656. doi: 10.1007/s00345-018-02619-0. Epub 2019 Jan 19. World J Urol. 2020. PMID: 30659302 Review.
Cited by
-
Structure, Inhibitors, and Biological Function in Nervous System and Cancer of Ubiquitin-Specific Protease 46.Bioinform Biol Insights. 2024 Oct 13;18:11779322241285982. doi: 10.1177/11779322241285982. eCollection 2024. Bioinform Biol Insights. 2024. PMID: 39410943 Free PMC article. Review.
-
Targeting sex steroid biosynthesis for breast and prostate cancer therapy.Nat Rev Cancer. 2023 Sep 8. doi: 10.1038/s41568-023-00609-y. Online ahead of print. Nat Rev Cancer. 2023. PMID: 37684402 Review. No abstract available.
-
Salinization Dramatically Enhance the Anti-Prostate Cancer Efficacies of AR/AR-V7 and Mnk1/2 Molecular Glue Degraders, Galeterone and VNPP433-3β Which Outperform Docetaxel and Enzalutamide in CRPC CWR22Rv1 Xenograft Mouse Model.Bioorg Chem. 2023 Oct;139:106700. doi: 10.1016/j.bioorg.2023.106700. Epub 2023 Jun 25. Bioorg Chem. 2023. PMID: 37392559 Free PMC article.
-
Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine.Acta Pharm Sin B. 2023 Feb;13(2):478-497. doi: 10.1016/j.apsb.2022.09.010. Epub 2022 Sep 21. Acta Pharm Sin B. 2023. PMID: 36873180 Free PMC article. Review.
-
Mechanisms and targeting of proteosome-dependent androgen receptor degradation in prostate cancer.Am J Clin Exp Urol. 2022 Dec 25;10(6):366-376. eCollection 2022. Am J Clin Exp Urol. 2022. PMID: 36636693 Free PMC article. Review.
References
-
- Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: current status and future prospects. The Prostate. 2004;61:332–353. - PubMed
-
- Vasaitis TS, Njar VC. Novel, potent anti-androgens of therapeutic potential: recent advances and promising developments. Future medicinal chemistry. 2010;2:667–680. - PubMed
-
- Shapiro D, Tareen B. Current and emerging treatments in the management of castration-resistant prostate cancer. Expert review of anticancer therapy. 2012;12:951–964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

