Optimized exosome isolation protocol for cell culture supernatant and human plasma
- PMID: 26194179
- PMCID: PMC4507751
- DOI: 10.3402/jev.v4.27031
Optimized exosome isolation protocol for cell culture supernatant and human plasma
Abstract
Extracellular vesicles represent a rich source of novel biomarkers in the diagnosis and prognosis of disease. However, there is currently limited information elucidating the most efficient methods for obtaining high yields of pure exosomes, a subset of extracellular vesicles, from cell culture supernatant and complex biological fluids such as plasma. To this end, we comprehensively characterize a variety of exosome isolation protocols for their efficiency, yield and purity of isolated exosomes. Repeated ultracentrifugation steps can reduce the quality of exosome preparations leading to lower exosome yield. We show that concentration of cell culture conditioned media using ultrafiltration devices results in increased vesicle isolation when compared to traditional ultracentrifugation protocols. However, our data on using conditioned media isolated from the Non-Small-Cell Lung Cancer (NSCLC) SK-MES-1 cell line demonstrates that the choice of concentrating device can greatly impact the yield of isolated exosomes. We find that centrifuge-based concentrating methods are more appropriate than pressure-driven concentrating devices and allow the rapid isolation of exosomes from both NSCLC cell culture conditioned media and complex biological fluids. In fact to date, no protocol detailing exosome isolation utilizing current commercial methods from both cells and patient samples has been described. Utilizing tunable resistive pulse sensing and protein analysis, we provide a comparative analysis of 4 exosome isolation techniques, indicating their efficacy and preparation purity. Our results demonstrate that current precipitation protocols for the isolation of exosomes from cell culture conditioned media and plasma provide the least pure preparations of exosomes, whereas size exclusion isolation is comparable to density gradient purification of exosomes. We have identified current shortcomings in common extracellular vesicle isolation methods and provide a potential standardized method that is effective, reproducible and can be utilized for various starting materials. We believe this method will have extensive application in the growing field of extracellular vesicle research.
Keywords: cancer; cell culture; exosomes; isolation; plasma; purification.
Figures


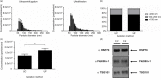
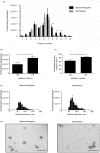
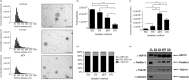

Similar articles
-
An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells.Stem Cell Res Ther. 2018 Jul 4;9(1):180. doi: 10.1186/s13287-018-0923-0. Stem Cell Res Ther. 2018. PMID: 29973270 Free PMC article.
-
Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses.Curr Protoc Cell Biol. 2020 Sep;88(1):e110. doi: 10.1002/cpcb.110. Curr Protoc Cell Biol. 2020. PMID: 32633898 Free PMC article.
-
Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches.Methods. 2015 Oct 1;87:46-58. doi: 10.1016/j.ymeth.2015.05.028. Epub 2015 Jun 2. Methods. 2015. PMID: 26044649
-
A review on comparative studies addressing exosome isolation methods from body fluids.Anal Bioanal Chem. 2023 Mar;415(7):1239-1263. doi: 10.1007/s00216-022-04174-5. Epub 2022 Jul 15. Anal Bioanal Chem. 2023. PMID: 35838769 Review.
-
Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics.Theranostics. 2020 Feb 19;10(8):3684-3707. doi: 10.7150/thno.41580. eCollection 2020. Theranostics. 2020. PMID: 32206116 Free PMC article. Review.
Cited by
-
High-throughput capture and in situ protein analysis of extracellular vesicles by chemical probe-based array.Nat Protoc. 2024 Oct 22. doi: 10.1038/s41596-024-01082-z. Online ahead of print. Nat Protoc. 2024. PMID: 39438698 Review.
-
Saponins from Panax notoginseng ameliorate steroid resistance in lupus nephritis through regulating lymphocyte-derived exosomes in mice.Front Pharmacol. 2022 Sep 23;13:946392. doi: 10.3389/fphar.2022.946392. eCollection 2022. Front Pharmacol. 2022. PMID: 36210823 Free PMC article.
-
Platelets and platelet extracellular vesicles in drug delivery therapy: A review of the current status and future prospects.Front Pharmacol. 2022 Oct 18;13:1026386. doi: 10.3389/fphar.2022.1026386. eCollection 2022. Front Pharmacol. 2022. PMID: 36330089 Free PMC article. Review.
-
Digital Decoding of Single Extracellular Vesicle Phenotype Differentiates Early Malignant and Benign Lung Lesions.Adv Sci (Weinh). 2022 Nov 17;10(1):e2204207. doi: 10.1002/advs.202204207. Online ahead of print. Adv Sci (Weinh). 2022. PMID: 36394090 Free PMC article.
-
Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications.ACS Biomater Sci Eng. 2021 Jun 14;7(6):2106-2149. doi: 10.1021/acsbiomaterials.1c00217. Epub 2021 May 14. ACS Biomater Sci Eng. 2021. PMID: 33988964 Free PMC article. Review.
References
-
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–8. - PubMed
-
- Wendler F, Bota-Rabassedas N, Franch-Marro X. Cancer becomes wasteful: emerging roles of exosomes(dagger) in cell-fate determination. J Extracell Vesicles. 2013;2:22390. doi: http://dx.doi.org/10.3402/jev.v2i0.22390. - DOI - PMC - PubMed
-
- EL Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. - PubMed
-
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. - PubMed
-
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

