Repurposing Kinase Inhibitors as Antiviral Agents to Control Influenza A Virus Replication
- PMID: 26192013
- PMCID: PMC4692129
- DOI: 10.1089/adt.2015.0003.drrr
Repurposing Kinase Inhibitors as Antiviral Agents to Control Influenza A Virus Replication
Abstract
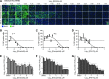
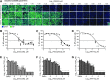
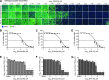
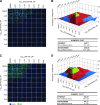
Influenza A virus (IAV) infection causes seasonal epidemics of contagious respiratory illness that causes substantial morbidity and some mortality. Regular vaccination is the principal strategy for controlling influenza virus, although vaccine efficacy is variable. IAV antiviral drugs are available; however, substantial drug resistance has developed to two of the four currently FDA-approved antiviral drugs. Thus, new therapeutic approaches are being sought to reduce the burden of influenza-related disease. A high-throughput screen using a human kinase inhibitor library was performed targeting an emerging IAV strain (H7N9) in A549 cells. The inhibitor library contained 273 structurally diverse, active cell permeable kinase inhibitors with known bioactivity and safety profiles, many of which are at advanced stages of clinical development. The current study shows that treatment of human A549 cells with kinase inhibitors dinaciclib, flavopiridol, or PIK-75 exhibits potent antiviral activity against H7N9 IAV as well as other IAV strains. Thus, targeting host kinases can provide a broad-spectrum therapeutic approach against IAV. These findings provide a path forward for repurposing existing kinase inhibitors safely as potential antivirals, particularly those that can be tested in vivo and ultimately for clinical use.
Figures

Similar articles
-
The clinically approved MEK inhibitor Trametinib efficiently blocks influenza A virus propagation and cytokine expression.Antiviral Res. 2018 Sep;157:80-92. doi: 10.1016/j.antiviral.2018.07.006. Epub 2018 Jul 7. Antiviral Res. 2018. PMID: 29990517
-
Chemical Genomics Approach Leads to the Identification of Hesperadin, an Aurora B Kinase Inhibitor, as a Broad-Spectrum Influenza Antiviral.Int J Mol Sci. 2017 Sep 8;18(9):1929. doi: 10.3390/ijms18091929. Int J Mol Sci. 2017. PMID: 28885544 Free PMC article.
-
The clinically licensed antifungal drug itraconazole inhibits influenza virus in vitro and in vivo.Emerg Microbes Infect. 2019;8(1):80-93. doi: 10.1080/22221751.2018.1559709. Emerg Microbes Infect. 2019. PMID: 30866762 Free PMC article.
-
Emerging cellular targets for influenza antiviral agents.Trends Pharmacol Sci. 2012 Feb;33(2):89-99. doi: 10.1016/j.tips.2011.10.004. Epub 2011 Dec 22. Trends Pharmacol Sci. 2012. PMID: 22196854 Review.
-
Screening methods for influenza antiviral drug discovery.Expert Opin Drug Discov. 2012 May;7(5):429-38. doi: 10.1517/17460441.2012.674510. Epub 2012 Mar 22. Expert Opin Drug Discov. 2012. PMID: 22435452 Review.
Cited by
-
Repositioning salicylanilide anthelmintic drugs to treat adenovirus infections.Sci Rep. 2019 Jan 9;9(1):17. doi: 10.1038/s41598-018-37290-3. Sci Rep. 2019. PMID: 30626902 Free PMC article.
-
Production of Inhalable Ultra-Small Particles for Delivery of Anti-Inflammation Medicine via a Table-Top Microdevice.Micromachines (Basel). 2022 Aug 25;13(9):1382. doi: 10.3390/mi13091382. Micromachines (Basel). 2022. PMID: 36144005 Free PMC article.
-
Ifenprodil and Flavopiridol Identified by Genomewide RNA Interference Screening as Effective Drugs To Ameliorate Murine Acute Lung Injury after Influenza A H5N1 Virus Infection.mSystems. 2019 Dec 10;4(6):e00431-19. doi: 10.1128/mSystems.00431-19. mSystems. 2019. PMID: 31822599 Free PMC article.
-
Repurposing host-based therapeutics to control coronavirus and influenza virus.Drug Discov Today. 2019 Mar;24(3):726-736. doi: 10.1016/j.drudis.2019.01.018. Epub 2019 Jan 31. Drug Discov Today. 2019. PMID: 30711575 Free PMC article. Review.
-
Kinome Profiling Identifies Druggable Targets for Novel Human Cytomegalovirus (HCMV) Antivirals.Mol Cell Proteomics. 2017 Apr;16(4 suppl 1):S263-S276. doi: 10.1074/mcp.M116.065375. Epub 2017 Feb 25. Mol Cell Proteomics. 2017. PMID: 28237943 Free PMC article.
References
-
- Fiore AE, Uyeki TM, Broder K, et al. : Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010;59:1–62 - PubMed
-
- Hoyert DL, Kung HC, Smith BL: Deaths: preliminary data for 2003. Natl Vital Stat Rep 2005;53:1–48 - PubMed
-
- Podewils LJ, Liedtke LA, McDonald LC, et al. : A national survey of severe influenza-associated complications among children and adults, 2003–2004. Clin Infect Dis 2005;40:1693–1696 - PubMed
-
- Gao R, Cao B, Hu Y, et al. : Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 2013;368:1888–1897 - PubMed
-
- Krammer F, Palese P: Universal influenza virus vaccines: need for clinical trials. Nat Immunol 2014;15:3–5 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

