LY2109761 enhances cisplatin antitumor activity in ovarian cancer cells
- PMID: 26191185
- PMCID: PMC4503057
LY2109761 enhances cisplatin antitumor activity in ovarian cancer cells
Abstract
Background and objective: Ovarian cancer is among the most lethal of all malignancies in women. While chemotherapy is the preferred treatment modality, chemoresistance severely limits treatment success. Because transforming growth factor-beta (TGF-β) could increase survival of ovarian cancer cells in the presence of cisplatin, we conducted a preclinical study of the antitumor effects of the TGF-β type I (TβRI) and type II (TβRII) kinase inhibitor LY2109761 in combination with cisplatin.
Methods: SKOV3, OV-90 and SKOV3(DDP) cells were treated with LY2109761, and/or cisplatin, and cell viability, apoptosis mRNA and protein expression levels were then evaluated. Furthermore, the efficacy of LY2109761 combined with cisplatin was further examined in established xenograft models.
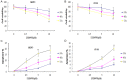
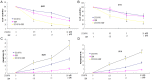
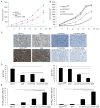
Results: LY2109761 was sufficient to induce spontaneous apoptosis of ovarian cancer cells. Combination with LY2109761 significantly augmented the cytotoxicity of cisplatin in both parental and cisplatin resistant ovarian cancer cells. LY2109761 significantly increased apoptotic cell death in cisplatin-resistant cells. Combination treatment of LY2109761 and cisplatin showed antiproliferative effects and induced a greater rate of apoptosis than the sum of the single-treatment rates and promoted tumor regression in established parental and cisplatin resistant ovarian cancer xenograft models.
Conclusions: Chemotherapeutic approaches using LY2109761 might enhance the treatment benefit of the cisplatin in the treatment of ovarian cancer patients.
Keywords: LY2109761; Ovarian cancer; chemotherapy; transforming growth factor-beta.
Figures

Similar articles
-
LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis.Mol Cancer Ther. 2008 Apr;7(4):829-40. doi: 10.1158/1535-7163.MCT-07-0337. Mol Cancer Ther. 2008. PMID: 18413796 Free PMC article.
-
Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma.Cancer Res. 2011 Dec 1;71(23):7155-67. doi: 10.1158/0008-5472.CAN-11-1212. Epub 2011 Oct 17. Cancer Res. 2011. PMID: 22006998
-
4-Acetylantroquinonol B suppresses autophagic flux and improves cisplatin sensitivity in highly aggressive epithelial cancer through the PI3K/Akt/mTOR/p70S6K signaling pathway.Toxicol Appl Pharmacol. 2017 Jun 15;325:48-60. doi: 10.1016/j.taap.2017.04.003. Epub 2017 Apr 10. Toxicol Appl Pharmacol. 2017. PMID: 28408137
-
Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway.Drug Des Devel Ther. 2015 Aug 10;9:4479-99. doi: 10.2147/DDDT.S86621. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26309397 Free PMC article. Review.
-
Clinical outcome of treatment with serine-threonine kinase inhibitors in recurrent epithelial ovarian cancer: a systematic review of literature.Expert Opin Investig Drugs. 2016 Jul;25(7):781-96. doi: 10.1080/13543784.2016.1181748. Epub 2016 May 13. Expert Opin Investig Drugs. 2016. PMID: 27101098 Free PMC article. Review.
Cited by
-
Shifting Paradigms for Suppressing Fibrosis in Kidney Transplants: Supplementing Perfusion Solutions With Anti-fibrotic Drugs.Front Med (Lausanne). 2022 Jan 10;8:806774. doi: 10.3389/fmed.2021.806774. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35083254 Free PMC article. Review.
-
A Positive Feedback Loop Between TGFβ and Androgen Receptor Supports Triple-negative Breast Cancer Anoikis Resistance.Endocrinology. 2021 Feb 1;162(2):bqaa226. doi: 10.1210/endocr/bqaa226. Endocrinology. 2021. PMID: 33294922 Free PMC article.
-
"DEPHENCE" system-a novel regimen of therapy that is urgently needed in the high-grade serous ovarian cancer-a focus on anti-cancer stem cell and anti-tumor microenvironment targeted therapies.Front Oncol. 2023 Jun 28;13:1201497. doi: 10.3389/fonc.2023.1201497. eCollection 2023. Front Oncol. 2023. PMID: 37448521 Free PMC article. Review.
-
Epithelial‑mesenchymal transition induced by bone morphogenetic protein 9 hinders cisplatin efficacy in ovarian cancer cells.Mol Med Rep. 2019 Mar;19(3):1501-1508. doi: 10.3892/mmr.2019.9814. Epub 2019 Jan 3. Mol Med Rep. 2019. PMID: 30628686 Free PMC article.
-
Evaluation of the cytotoxic effect of Ly2109761 on HeLa cells using the xCELLigence RTCA system.Oncol Lett. 2019 Jan;17(1):683-687. doi: 10.3892/ol.2018.9556. Epub 2018 Oct 9. Oncol Lett. 2019. PMID: 30655817 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA. 2012;62:10–29. - PubMed
-
- Sudo T. Molecular-targeted therapies for ovarian cancer: prospects for the future. Int J Clin Oncol. 2012;17:424–429. - PubMed
-
- McKeage MJ. New-generation platinum drugs in the treatment of cisplatin-resistant cancers. Expert Opin Investig Drugs. 2005;14:1033–46. - PubMed
-
- Tewari K, Mehta R, Burger R, et al. Emerging drugs for ovarian cancer. Expert Opin Emerg Drugs. 2005;10:413–42. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
