Leukemia inhibitory factor (LIF)
- PMID: 26187859
- PMCID: PMC4581962
- DOI: 10.1016/j.cytogfr.2015.07.001
Leukemia inhibitory factor (LIF)
Abstract
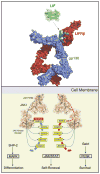
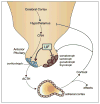
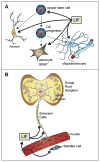
Leukemia inhibitory factor (LIF) is the most pleiotropic member of the interleukin-6 family of cytokines. It utilises a receptor that consists of the LIF receptor β and gp130 and this receptor complex is also used by ciliary neurotrophic growth factor (CNTF), oncostatin M, cardiotrophin1 (CT1) and cardiotrophin-like cytokine (CLC). Despite common signal transduction mechanisms (JAK/STAT, MAPK and PI3K) LIF can have paradoxically opposite effects in different cell types including stimulating or inhibiting each of cell proliferation, differentiation and survival. While LIF can act on a wide range of cell types, LIF knockout mice have revealed that many of these actions are not apparent during ordinary development and that they may be the result of induced LIF expression during tissue damage or injury. Nevertheless LIF does appear to have non-redundant actions in maternal receptivity to blastocyst implantation, placental formation and in the development of the nervous system. LIF has also found practical use in the maintenance of self-renewal and totipotency of embryonic stem cells and induced pluripotent stem cells.
Keywords: Embryonic stem cells; JAK/STAT/SOCS; LIF receptor; Leukemia inhibitory factor; Nerve and muscle; Pregnancy.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest: NAN is an inventor on patents relating to LIF
Figures

Similar articles
-
Stimulation of the JAK/STAT pathway by LIF and OSM in the human granulosa cell line COV434.J Reprod Immunol. 2015 Apr;108:48-55. doi: 10.1016/j.jri.2015.03.002. Epub 2015 Mar 16. J Reprod Immunol. 2015. PMID: 25817464
-
Leukemia inhibitory factor signaling in Xenopus embryo: Insights from gain of function analysis and dominant negative mutant of the receptor.Dev Biol. 2019 Mar 15;447(2):200-213. doi: 10.1016/j.ydbio.2018.12.020. Epub 2018 Dec 19. Dev Biol. 2019. PMID: 30578761
-
Contributions of leukemia inhibitory factor receptor and oncostatin M receptor to signal transduction in heterodimeric complexes with glycoprotein 130.J Immunol. 1999 Dec 15;163(12):6651-8. J Immunol. 1999. PMID: 10586060
-
Role of leukemia inhibitory factor during mammalian development.Eur Cytokine Netw. 1996 Dec;7(4):699-712. Eur Cytokine Netw. 1996. PMID: 9010672 Review.
-
The Multifaceted Actions of Leukaemia Inhibitory Factor in Mediating Uterine Receptivity and Embryo Implantation.Am J Reprod Immunol. 2016 Mar;75(3):246-55. doi: 10.1111/aji.12474. Epub 2016 Jan 28. Am J Reprod Immunol. 2016. PMID: 26817565 Review.
Cited by
-
Dysfunction and ceRNA network of the tumor suppressor miR-637 in cancer development and prognosis.Biomark Res. 2022 Sep 30;10(1):72. doi: 10.1186/s40364-022-00419-8. Biomark Res. 2022. PMID: 36175921 Free PMC article. Review.
-
Super-Enhancer Driven LIF/LIFR-STAT3-SOX2 Regulatory Feedback Loop Promotes Cancer Stemness in Head and Neck Squamous Cell Carcinoma.Adv Sci (Weinh). 2024 Oct;11(40):e2404476. doi: 10.1002/advs.202404476. Epub 2024 Aug 29. Adv Sci (Weinh). 2024. PMID: 39206755 Free PMC article.
-
Stüve-Wiedemann Syndrome: Update on Clinical and Genetic Aspects.Mol Syndromol. 2016 Apr;7(1):12-8. doi: 10.1159/000444729. Epub 2016 Mar 16. Mol Syndromol. 2016. PMID: 27194968 Free PMC article. Review.
-
Possible Role of Leukemia Inhibitory Factor and Inflammatory Cytokines in The Recurrent Spontaneous Abortion: A Case-Control Study.Int J Fertil Steril. 2023 Feb 1;17(2):140-144. doi: 10.22074/ijfs.2022.548425.1258. Int J Fertil Steril. 2023. PMID: 36906832 Free PMC article.
-
The Role of Interleukin-6 Family Members in Cardiovascular Diseases.Front Cardiovasc Med. 2022 Mar 23;9:818890. doi: 10.3389/fcvm.2022.818890. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35402550 Free PMC article. Review.
References
-
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–90. - PubMed
-
- Moreau JF, Bonneville M, Godard A, Gascan H, Gruart V, Moore MA, et al. Characterization of a factor produced by human T cell clones exhibiting eosinophil-activating and burst-promoting activities. Journal of immunology. 1987;138:3844–9. - PubMed
-
- Baumann H, Onorato V, Gauldie J, Jahreis GP. Distinct sets of acute phase plasma proteins are stimulated by separate human hepatocyte-stimulating factors and monokines in rat hepatoma cells. The Journal of biological chemistry. 1987;262:9756–68. - PubMed
-
- Patterson PH, Chun LL. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. I. Effects of conditioned medium. Developmental biology. 1977;56:263–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

