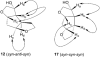Enantioselective Synthesis of Dioxatriquinane Structural Motifs for HIV-1 Protease Inhibitors Using a Cascade Radical Cyclization
- PMID: 26185337
- PMCID: PMC4500529
- DOI: 10.1016/j.tetlet.2015.01.019
Enantioselective Synthesis of Dioxatriquinane Structural Motifs for HIV-1 Protease Inhibitors Using a Cascade Radical Cyclization
Abstract
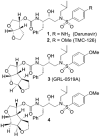
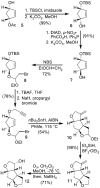
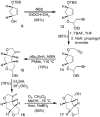
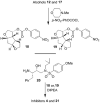
Synthesis of novel HIV-1 protease inhibitors incorporating dioxatriquinane-derived P2-ligands is described. The tricyclic ligand alcohol contains five contiguous chiral centers. The ligand alcohols were prepared in optically active form by an enzymatic asymmetrization of mesodiacetate, cascade radical cyclization, and Lewis acid catalyzed reduction as the key steps. Inhibitors with dioxatriquinane-derived P2-ligands exhibited low nanomolar HIV-1 protease activity.
Keywords: Cascade; Dioxatriquinane; HIV-1 protease; Inhibitor; Radical.
Figures
Similar articles
-
Structure-based design of novel HIV-1 protease inhibitors to combat drug resistance.J Med Chem. 2006 Aug 24;49(17):5252-61. doi: 10.1021/jm060561m. J Med Chem. 2006. PMID: 16913714
-
An Enzymatic Route to the Synthesis of Tricyclic Fused Hexahydrofuranofuran P2-Ligand for a Series of Highly Potent HIV-1 Protease Inhibitors.Tetrahedron Lett. 2024 Apr 28;140:155013. doi: 10.1016/j.tetlet.2024.155013. Epub 2024 Mar 16. Tetrahedron Lett. 2024. PMID: 38586565
-
Design and synthesis of highly potent HIV-1 protease inhibitors with novel isosorbide-derived P2 ligands.Bioorg Med Chem Lett. 2014 Jun 1;24(11):2465-8. doi: 10.1016/j.bmcl.2014.04.008. Epub 2014 Apr 13. Bioorg Med Chem Lett. 2014. PMID: 24767846
-
Structure-based design: synthesis and biological evaluation of a series of novel cycloamide-derived HIV-1 protease inhibitors.J Med Chem. 2005 May 19;48(10):3576-85. doi: 10.1021/jm050019i. J Med Chem. 2005. PMID: 15887965
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
Cited by
-
Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS.J Med Chem. 2016 Jun 9;59(11):5172-208. doi: 10.1021/acs.jmedchem.5b01697. Epub 2016 Jan 22. J Med Chem. 2016. PMID: 26799988 Free PMC article. Review.
-
Nature Inspired Molecular Design: Stereoselective Synthesis of Bicyclic and Polycyclic Ethers for Potent HIV-1 Protease Inhibitors.Asian J Org Chem. 2018 Aug;7(8):1448-1466. doi: 10.1002/ajoc.201800255. Epub 2018 Jun 8. Asian J Org Chem. 2018. PMID: 31595212 Free PMC article.
-
Catalysis-Enabled Concise and Stereoselective Total Synthesis of the Tricyclic Prostaglandin D2 Metabolite Methyl Ester.Angew Chem Int Ed Engl. 2022 Jan 26;61(5):e202115633. doi: 10.1002/anie.202115633. Epub 2021 Dec 14. Angew Chem Int Ed Engl. 2022. PMID: 34870881 Free PMC article.
-
Photoinduced Cycloadditions in the Diversity-Oriented Synthesis Toolbox: Increasing Complexity with Straightforward Postphotochemical Modifications.Aust J Chem. 2015;68(11):1672-1681. doi: 10.1071/CH15266. Epub 2015 Jul 24. Aust J Chem. 2015. PMID: 29249834 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources