Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression
- PMID: 26181207
- PMCID: PMC4650738
- DOI: 10.1038/cddis.2015.193
Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression
Abstract
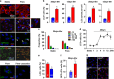
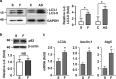
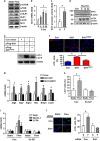
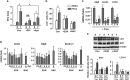
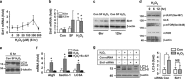
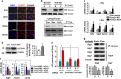
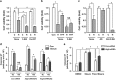
Disturbed cell autophagy is found in various cardiovascular disease conditions. Biomechanical stimuli induced by laminar blood flow have important protective actions against the development of various vascular diseases. However, the impacts and underlying mechanisms of shear stress on the autophagic process in vascular endothelial cells (ECs) are not entirely understood. Here we investigated the impacts of shear stress on autophagy in human vascular ECs. We found that shear stress induced by laminar flow, but not that by oscillatory or low-magnitude flow, promoted autophagy. Time-course analysis and flow cessation experiments confirmed that this effect was not a transient adaptive stress response but appeared to be a sustained physiological action. Flow had no effect on the mammalian target of rapamycin-ULK pathway, whereas it significantly upregulated Sirt1 expression. Inhibition of Sirt1 blunted shear stress-induced autophagy. Overexpression of wild-type Sirt1, but not the deacetylase-dead mutant, was sufficient to induce autophagy in ECs. Using both of gain- and loss-of-function experiments, we showed that Sirt1-dependent activation of FoxO1 was critical in mediating shear stress-induced autophagy. Shear stress also induced deacetylation of Atg5 and Atg7. Moreover, shear stress-induced Sirt1 expression and autophagy were redox dependent, whereas Sirt1 might act as a redox-sensitive transducer mediating reactive oxygen species-elicited autophagy. Functionally, we demonstrated that flow-conditioned cells are more resistant to oxidant-induced cell injury, and this cytoprotective effect was abolished after inhibition of autophagy. In summary, these results suggest that Sirt1-mediated autophagy in ECs may be a novel mechanism by which laminar flow produces its vascular-protective actions.
Figures
Similar articles
-
Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against atherosclerosis.Cell Death Dis. 2020 Feb 21;11(2):141. doi: 10.1038/s41419-020-2343-1. Cell Death Dis. 2020. PMID: 32081881 Free PMC article.
-
Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage.Autophagy. 2012 May 1;8(5):812-25. doi: 10.4161/auto.19471. Epub 2012 May 1. Autophagy. 2012. PMID: 22622204
-
Association of SIRT1 expression with shear stress induced endothelial progenitor cell differentiation.J Cell Biochem. 2012 Dec;113(12):3663-71. doi: 10.1002/jcb.24239. J Cell Biochem. 2012. PMID: 22740055
-
Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer.Curr Neurovasc Res. 2017;14(3):299-304. doi: 10.2174/1567202614666170718092010. Curr Neurovasc Res. 2017. PMID: 28721811 Free PMC article. Review.
-
mTOR, AMPK, and Sirt1: Key Players in Metabolic Stress Management.Crit Rev Eukaryot Gene Expr. 2015;25(1):59-75. doi: 10.1615/critreveukaryotgeneexpr.2015012975. Crit Rev Eukaryot Gene Expr. 2015. PMID: 25955819 Review.
Cited by
-
Cell adhesion molecule IGPR-1 activates AMPK connecting cell adhesion to autophagy.J Biol Chem. 2020 Dec 4;295(49):16691-16699. doi: 10.1074/jbc.RA120.014790. Epub 2020 Sep 25. J Biol Chem. 2020. PMID: 32978258 Free PMC article.
-
Proteomic analysis reveals the aging-related pathways contribute to pulmonary fibrogenesis.Aging (Albany NY). 2023 Dec 22;15(24):15382-15401. doi: 10.18632/aging.205355. Epub 2023 Dec 22. Aging (Albany NY). 2023. PMID: 38147026 Free PMC article.
-
Recent advances in cerebral cavernous malformation research.Vessel Plus. 2018;2:21. doi: 10.20517/2574-1209.2018.34. Epub 2018 Aug 28. Vessel Plus. 2018. PMID: 31360916 Free PMC article.
-
Biphasic effects of autophagy on decompression bubble-induced endothelial injury.J Cell Mol Med. 2019 Dec;23(12):8058-8066. doi: 10.1111/jcmm.14672. Epub 2019 Sep 12. J Cell Mol Med. 2019. PMID: 31515946 Free PMC article.
-
Healthy versus Unhealthy Adipose Tissue Expansion: the Role of Exercise.J Obes Metab Syndr. 2022 Mar 30;31(1):37-50. doi: 10.7570/jomes21096. J Obes Metab Syndr. 2022. PMID: 35283364 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

